
MiSeq
System Guide
ILLUMINA PROPRIETARY
Document # 1000000061014 v00
July 2018
For Research Use Only. Not
for use in diagnostic procedures.
This document and its contents are proprietary to Illumina, Inc. and its affiliates ("Illumina"), and are intended solely for
the contractual use of its customer in connection with the use of the product(s) described herein and for no other
purpose. This document and its contents shall not be used or distributed for any other purpose and/or otherwise
communicated, disclosed, or reproduced in any way whatsoever without the prior written consent of Illumina. Illumina
does not convey any license under its patent, trademark, copyright, or common-law rights nor similar rights of any third
parties by this document.
The instructions in this document must be strictly and explicitly followed by qualified and properly trained personnel in
order to ensure the proper and safe use of the product(s) described herein. All of the contents of this document must be
fully read and understood prior to using such product(s).
FAILURE TO COMPLETELY READ AND EXPLICITLY FOLLOW ALL OF THE INSTRUCTIONS CONTAINED HEREIN MAY
RESULT IN DAMAGE TO THE PRODUCT(S), INJURY TO PERSONS, INCLUDING TO USERS OR OTHERS, AND DAMAGE
TO OTHER PROPERTY, AND WILL VOID ANY WARRANTY APPLICABLE TO THE PRODUCT(S).
ILLUMINA DOES NOT ASSUME ANY LIABILITY ARISING OUT OF THE IMPROPER USE OF THE PRODUCT(S)
DESCRIBED HEREIN (INCLUDING PARTS THEREOF OR SOFTWARE).
© 2018 Illumina, Inc. All rights reserved.
All trademarks are the property of Illumina, Inc. or their respective owners. For specific trademark information, see
www.illumina.com/company/legal.html.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
ii
MiSeq System Guide

Table of Contents
Chapter 1 Overview 1
Introduction 1
Additional Resources 1
Components 2
MiSeq Concepts 4
System Software 5
Secondary Analysis Options 7
Sequencing Analysis Viewer 8
Required Disk Space 8
MiSeq Reagent Kit Overview 9
Chapter 2 Getting Started 13
Start the MiSeq 13
Customize System Settings 13
Configure Notifications of BaseSpace Updates 14
Set Email Preferences 14
Set Default Folder Locations 14
User-Supplied Consumables 15
Chapter 3 Sequencing 17
Introduction 17
Run Duration 17
MiSeq Workflow 18
Thaw Reagent Cartridge 19
Inspect the Reagent Cartridge 19
Denature and Dilute Libraries 20
Load Sample Libraries 20
Set Up a Run Using MCS 20
Clean the Flow Cell 21
Load the Flow Cell 22
Load Reagents 23
Starting the Run 25
Monitor the Run 26
Perform a Post-Run Wash 28
Chapter 4 Maintenance 33
Maintenance Frequency 33
Maintenance Frequency for the VeriSeq PGS Workflow 33
Perform a Maintenance Wash 34
Perform a Standby Wash 36
Manage Files 38
Software Updates 39
Shut Down the Instrument 39
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
iii

Appendix A Troubleshooting 41
Introduction 41
Bundle Logs for Troubleshooting 41
Perform a System Check 42
Pause or Stop a Run 42
Raise Reagent Cartridge Sippers Manually 44
Resolve Run Setup Errors 44
Resolve RFID Read Failure 44
Perform a Volume Test 45
Measure Expected Wash Volumes 46
Configure System Settings 46
Appendix B Output Files and Folders 49
Run Folders 49
MiSeqOutput Folder Contents 49
RTA Folders and Files 51
Index 53
Technical Assistance 57
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
iv
MiSeq System Guide

Chapter 1 Overview
Introduction 1
Additional Resources 1
Components 2
MiSeq Concepts 4
System Software 5
Secondary Analysis Options 7
Sequencing Analysis Viewer 8
Required Disk Space 8
MiSeq Reagent Kit Overview 9
Introduction
The Illumina
®
MiSeq
®
system combines proven sequencing by synthesis (SBS) technology with a
revolutionary workflow that lets you go from DNA to analyzed data in as few as eight hours. The MiSeq
integrates cluster generation, sequencing, and data analysis on a single instrument.
Features
u Walk away automation—After setting up your run, which includes loading the pre-filled reagent cartridge,
buffer bottle, and flow cell, no additional hands-on time is required.
u Prefilled reagent cartridge—A specially designed single-use prefilled reagent cartridge provides reagents
for cluster generation and sequencing, including paired-end sequencing reagents and indexing reagents.
Integrated radio-frequency identification (RFID) tracking enables accurate consumable tracking.
u Interface controls—The MiSeq Control Software (MCS) interface provides controls to configure the
instrument, set up and monitor runs, and perform maintenance procedures.
u Convenient flow cell loading—A clamping mechanism auto-positions the flow cell as it is loaded onto the
instrument. Integrated radio-frequency identification (RFID) tracking enables accurate consumable
tracking.
u Innovative fluidics architecture—The MiSeq fluidics system enables unmatched efficiency in chemistry
cycle time during sequencing.
u Real-time analysis (RTA)—Integrated analysis software performs real-time on-instrument data analysis
during the sequencing run, which includes image analysis and base calling, and saves valuable
downstream analysis time.
u Integrated secondary analysis software— MiSeq Reporter software processes data from analysis by RTA
to perform alignment and provides information about each sample analyzed.
Additional Resources
The MiSeq system support pages on the Illumina website provide additional resources. These resources
include software, training, compatible products, and the following documentation. Always check support
pages for the latest versions.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
1

Resource Description
MiSeq System Site Prep
Guide (document #
15027615)
Provides specifications for laboratory space, electrical requirements, and environmental
considerations.
MiSeq System Safety and
Compliance Guide
(document # 15027616)
Provides information about instrument labeling, compliance certifications, and safety
considerations.
Illumina Experiment
Manager User Guide
(document # 15031335)
Provides instructions for creating sample plates and sample sheets for different workflows
and library types.
BlueFuse Workflow
Manager User Guide
(document #
1000000028842)
Provides instructions for creating sample plates and sample sheets for use with the
VeriSeq PGS workflow.
MiSeq Sample Sheet
Quick Reference Guide
(document # 15028392)
Provides information about adding sample sheet settings to your sample sheet.
MiSeq System Denature
and Dilute Libraries Guide
(document # 15039740)
Provides instructions for denaturing and diluting prepared sample libraries before
sequencing on the MiSeq, and preparing a PhiX control. This step applies to most library
types.
MiSeq Custom Primers
Guide (document #
15041638)
Provides instructions for preparing and loading custom primers, and editing the samples
sheet for custom primers.
MiSeq ReporterUser Guide
(document # 15042295)
Provides a comprehensive overview of analysis procedures, analysis workflows, and
output files generated by MiSeq Reporter, as well as computing requirements, off-
instrument installation instructions, and troubleshooting information.
MiSeq ReporterOnline Help
Provides instructions for using the MiSeq Reporter software.
BlueFuse Multi Software
Guide (document #
15053620)
Provides a comprehensive overview of analysis procedures, analysis workflows, and files
generated by BlueFuse Multi, as well as computing requirements, and troubleshooting
information. Use this guide with the VeriSeq PGS workflow.
BaseSpace User Guide
(document # 15044182)
Provides instructions for using BaseSpace and descriptions of the graphs generated for
each analysis workflow.
BaseSpace Onsite System
Guide (document #
15049148)
Provides instructions for using the BaseSpace Onsite System.
Components
The MiSeq comprises a touch screen monitor, a status bar, a power button with adjacent USBports, and
three compartments.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
2
MiSeq System Guide

A Flow cell compartment—Contains the flow cell stage that houses the flow cell throughout the run. Flow cell
stage motors move the stage out of the enclosed optical module for flow cell loading and returns the stage
when the run begins.
B Enclosed optics compartment—Contains optical components that enable imaging of the flow cell.
C Status bar—Indicates flow cell status as ready to sequence (green), processing (blue), or needs attention
(orange).
D Touch screen monitor—Displays the control software interface for system configuration and run setup.
E External USB ports—Facilitates the transfer of files and data to the instrument computer from the touch
screen monitor.
F Reagent compartment—Contains reagents at proper temperatures, wash solutions, and a bottle for used
reagents. A magnetic latch secures the reagent compartment door.
The MiSeq interface guides you through the run setup steps using the touch screen monitor. Loading run
components requires access to the reagent compartment and the flow cell compartment.
Flow Cell Compartment
The flow cell compartment contains the flow cell stage, thermal station, and fluidics connections to the flow
cell. The flow cell stage holds the flow cell and the flow cell clamp secures and positions the flow cell. When
the flow cell clamp closes, two pins near the clamp hinge auto-position the flow cell.
The thermal station, located beneath the flow cell stage, controls changes in flow cell temperature required
for cluster generation and sequencing.
Figure 1 Flow Cell Compartment
A Flow Cell Stage
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
3
MiSeq System Guide

B Flow Cell Compartment Door
C Flow Cell Clamp
D Flow Cell
E Flow Cell Clamp Release Button
Reagent Compartment
The reagent compartment contains the reagent chiller, and positions for the wash buffer (PR2) bottle and the
waste bottle. To maintain a consistent temperature, open and close the reagent chiller only when instructed.
NOTE
The required temperature range of the reagent chiller is 2°C to 11°C.
During the run, the reagent chiller holds a single-use reagent cartridge. During the instrument wash, the
reagent chiller holds the wash tray. The software automatically lowers sippers into each reservoir of the
reagent cartridge at the appropriate time during a run depending on the process being performed.
To the right of the reagent chiller are form-fitted slots for thePR2 bottle and the waste bottle. The sipper
handle locks the bottles in place and lowers the appropriate sipper into each bottle. Reagents are pumped
through the sippers and fluidics lines, and then to the flow cell. Reagent waste is delivered to the waste bottle
throughout the process.
Figure 2 Reagent Compartment Components
A Reagent Chiller
B Sipper Handle (shown in raised position)
C PR2 Bottle
D Waste Bottle
E Reagent Cartridge
MiSeq Concepts
The following concepts and terms are common to the run setup steps on the MiSeq.
Concept Description
Analysis Workflow A secondary analysis procedure is performed by MiSeq Reporter. The analysis workflow
for each run is specified in the sample sheet or by the selected module.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
4
MiSeq System Guide

Concept Description
Manifest The file that specifies a reference genome and targeted reference regions to be used in
the alignment step. For workflows that require a manifest, the manifest file is specified in
the sample sheet and copied to the manifest folder designated in MCS.
Reference Genome A FASTA format file that contains the genome sequences used during analysis. For most
analysis workflows, the reference genome file is specified in the sample sheet.
Run Folder The folder structure populated by RTA software (MiSeqOutput folder) or the folder
populated by MiSeq Reporter (MiSeqAnalysis). For more information, see
Run Folders
on
page 49.
Sample Sheet A comma-separated values file (*.csv) that contains information used to set up and
analyze a sequencing run, including a list of samples and their index sequences.
The sample sheet must be provided during the run setup steps on the MiSeq. After the
run begins, the sample sheet is renamed to SampleSheet.csv and copied to the run
folders: MiSeqTemp, MiSeqOutput, and MiSeqAnalysis.
For more information on analysis workflows and manifest file formats, see the
MiSeq Reporter Software Guide
(document #15042295)
.
For more information on sample sheets, see the
MiSeq Sample Sheet Quick Reference Guide (document #
15028392)
.
System Software
The instrument software suite includes integrated applications that perform sequencing runs, on-instrument
analysis, and related functions.
u MiSeq Control Software (MCS)—Controls instrument operation. The MiSeq Control Software (MCS)
interface guides you through the steps to load the flow cell and reagents before beginning the run. An
overview of quality statistics appears as the run progresses.
u During the run, MCS operates the flow cell stage, dispenses reagents, controls flow cell temperatures,
and captures images of clusters on the flow cell. MCS performs the run according to parameters
specified in the sample sheet.
u Real-time analysis (RTA) software—Performs image analysis and base calling, and assigns a quality score
to each base for each cycle. Images are temporarily stored in the run folder for processing by RTA, and
then automatically deleted when analysis by RTA is complete.
u Integrated secondary analysis software—Performs secondary analysis. MiSeq Reporter processes base
calls generated by the RTA software, and produces information about alignment, variants, and contig
assemblies for each genome requested. The analysis workflow specified in the sample sheet determines
the type of analysis performed. For more information, see
MiSeq Reporter Software
on page 8.
Optional software used off-instrument includes the Sequencing Analysis Viewer (SAV). For more information,
see
Sequencing Analysis Viewer
on page 8.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
5
MiSeq System Guide

Status Icons
A status icon on the control software interface indicates a change in conditions during run setup or during the
run. A number on the icon indicates the number of conditions for a status.
When a run status changes, the icon blinks to alert you. Select the icon to view a description of the condition.
Select Acknowledge to clear the message, and then Close to close the dialog box.
Filter the types of messages that appear in the status window by selecting the icons along the top margin of
the window. Selecting an icon toggles the condition to show or hide.
Status
Icon
Status
Name
Description
Status
OK
No change. System is normal.
Attention Important information. Action is recommended.
Warning Warnings do not stop a run. However, some warnings require action before proceeding.
Error Errors usually stop a run and generally require action before proceeding with the run.
Activity Indicators
An activity indicator icon displays the activity the instrument is currently performing.
Figure 3 Activity Indicators
From left to right, the activity indicators represent the following activities:
u Moving the Y-stage
u Moving the Z-stage
u Activating electronics functionality
u Using the camera
u Pumping through the fluidics system
Sensor Indicators
Sensor indicators, which appear at the base of each interface screen, represent the status of instrument
components.
Figure 4 Sensor Indicators
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
6
MiSeq System Guide

From left to right, the sensor indicators represent the following components:
u Flow cell compartment door in the closed or open positions
u Temperature of the reagent chiller in °C
u Temperature of the flow cell in °C
u Status of BaseSpace
®
connection (not connected shown)
Secondary Analysis Options
MiSeq sequencing data can be analyzed on the instrument computer using MiSeq Reporter, on a networked
server using BaseSpace
™
Onsite, or on the cloud using BaseSpace. These applications produce information
about alignment, variants, and contig assemblies for each genome requested and for each sample of a
multisample run. If performing the VeriSeq
™
PGS workflow, use BlueFuse
™
Multi software for analysis.
BaseSpace and BaseSpace Onsite Overview
BaseSpace is the Illumina cloud computing environment. BaseSpace Onsite provides a computing
environment on a dedicated server, complete with run setup tools and analysis options.
Log in to BaseSpace or BaseSpace Onsite when you set up the sequencing run. When using BaseSpace or
BaseSpace Onsite, you have the additional option to store run data locally. For more information, see
Customize System Settings
on page 13.
When you begin your sequencing run, the icon changes to indicate that the MiSeq is connected to
BaseSpace or BaseSpace Onsite and data files are being transferred to the specified location.
Figure 5 Connected to BaseSpace Icon
Figure 6 Connected to BaseSpace Onsite Icon
Using BaseSpace, data files are encrypted in transit, decrypted during analysis, and encrypted again when
stored. Using BaseSpace Onsite, data files are encrypted in transit, decrypted during analysis, and can be
optionally encrypted again when stored.
BaseSpace and BaseSpace Onsite automatically disconnect from the MiSeq at the end of the run or as soon
as all RTA analysis files have finished transfer. If the internet connection is interrupted, analysis files continue
to upload after the connection is restored from the point when the interruption occurred.
As soon as the last base call file is uploaded to BaseSpace or BaseSpace Onsite, secondary analysis of your
data begins. The same analysis workflows are supported on BaseSpace and BaseSpace Onsite as with on-
instrument analysis using MiSeq Reporter.
For MiSeq Reporter, several genomes are provided during installation. BaseSpace and BaseSpace Onsite
only support genomes included with MiSeq Reporter.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
7
MiSeq System Guide

You can connect to BaseSpace at basespace.illumina.com. Log in using your MyIllumina account login. For
more information on BaseSpace, see the
BaseSpace User Guide (document # 15044182)
and the
BaseSpace support pages on the Illumina website.
For more information on BaseSpace Onsite, see the
BaseSpace Onsite System Guide (document #
15049148)
and the BaseSpace OnSite support pages on the Illumina website.
MiSeq Reporter Software
MiSeq Reporter is a Windows Service application that processes base calls generated by RTA software.
MiSeq Reporter begins secondary analysis immediately after the completion of analysis of the sequencing run
by the RTA software.
MiSeq Reporter runs on the instrument computer. However, the software interface must be viewed through a
web browser on another computer that is connected to the same network as the MiSeq Reporter.
When secondary analysis is complete, a file named CompletedJobInfo.xml is written to the run folder. For
more information, see the
MiSeq Reporter Software Guide (document #15042295)
.
Sequencing During Analysis
The MiSeq system computing resources are dedicated to either sequencing or analysis.
With MiSeq Reporter, if a new sequencing run is started on the MiSeq before secondary analysis of an earlier
run is complete, MiSeq Reporter analysis is automatically stopped.
To restart MiSeq Reporter, use the Requeue feature after the new sequencing run is complete.
After the new run completes sequencing, secondary analysis of the earlier run automatically starts again from
the beginning.
Sequencing Analysis Viewer
You can monitor your run in greater detail without interfering with the run using the Illumina Sequencing
Analysis Viewer (SAV). Your MiSeq must be networked to view primary analysis results with SAV.
SAV allows you to review metrics during a run as metrics are generated, and later after a run has completed.
Install SAV onto a computer independent of the MiSeq with access to the same network connected to the
instrument. After launching the software, browse to the output folder for your run.
After template generation, SAV provides metrics generated by RTA and organizes the metrics into plots,
graphs, and tables.
NOTE
SAV is universal to Illumina sequencing systems, most of which use an 8-lane flow cell. Some views include
drop-down lists showing lanes 1–8. Because the MiSeq flow cell has a single lane, select All or Lane 1. For
more information, see the
Sequencing Analysis Viewer User Guide (document # 15020619)
.
Required Disk Space
The integrated instrument computer has approximately 550GB of storage capacity.
Before starting a run, the software checks available disk space. If there is not enough disk space for the run,
a message indicating how much disk space is required appears.
If prompted to make disk space available, move or delete older run folders as appropriate. For more
information, see
Manage Files
on page 38. After clearing adequate disk space, select Restart Check.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
8
MiSeq System Guide

MiSeq Reagent Kit Overview
The MiSeq Reagent Kit is a single-use reagent kit required to perform a sequencing run. It is available in
different types and sizes. Each type of MiSeq Reagent Kit includes a kit-specific flow cell type and all
reagents required for performing a run.
The flow cell, PR2 bottle, and reagent cartridge provided in the kit use radio-frequency identification (RFID)
for accurate consumable tracking and compatibility.
Always use the reagent cartridge associated with your flow cell type. If the reagent cartridge is not
compatible, a message appears during run setup that prompts you to load a compatible reagent cartridge.
For a description of available reagent kits, visit the MiSeq Reagent Kits product page on the Illumina website.
Flow Cell
The MiSeq flow cell is a single-use glass-based substrate where clusters are generated and the sequencing
reaction is performed.
Reagents enter the flow cell through the inlet port, pass through the single-lane imaging area, and then exit
the flow cell through the outlet port. Waste exiting the flow cell is delivered to the waste bottle.
Libraries are loaded onto the reagent cartridge before setting up the run, and then automatically transferred
to the flow cell after the run begins.
Figure 7 MiSeq Flow Cell
A Outlet Port
B Imaging Area
C Inlet Port
Flow Cell Cap Color
The cap color of the flow cell container indicates the flow cell type:
Flow Cell Flow Cell Cap Color
Standard Flow Cell
PGS Flow Cell
Clear
Micro Flow Cell Green
Nano Flow Cell Yellow
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
9
MiSeq System Guide

Reagent Cartridge Overview
The MiSeq reagent cartridge is a single-use consumable consisting of foil-sealed reservoirs prefilled with
clustering and sequencing reagents sufficient for sequencing one flow cell.
Each reservoir on the cartridge is numbered. Sample libraries are loaded onto the cartridge in position 17,
which is labeled Load Samples.
WARNING
This set of reagents contains potentially hazardous chemicals. Personal injury can occur through inhalation,
ingestion, skin contact, and eye contact. Wear protective equipment, including eye protection, gloves, and
laboratory coat appropriate for risk of exposure. Handle used reagents as chemical waste and discard in
accordance with applicable regional, national, and local laws and regulations. For additional environmental,
health, and safety information, see the SDS at support.illumina.com/sds.html.
Reserved Reservoirs
Figure 8 Reagent Cartridge with Numbered Reservoirs
Position Reagent Name Description
8 LDR Denaturation Reagent (contains formamide)
17 Reserved Load Sample (Reserved for sample libraries)
Table 1 Reagent Cartridge Reservoirs
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
10
MiSeq System Guide

Position Reagent Name Description
18 Reserved Reserved for custom Read 1 primer [Optional]
19 Reserved Reserved for custom Index Read primer [Optional]
20 Reserved Reserved for custom Read 2 primer [Optional]
NOTE
For more information about using custom primers on the MiSeq reagent cartridge, see the
MiSeq Custom
Primers Guide (document # 15041638)
.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
11
MiSeq System Guide
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
12
MiSeq System Guide

Chapter 2 Getting Started
Start the MiSeq 13
Customize System Settings 13
Configure Notifications of BaseSpace Updates 14
Set Email Preferences 14
Set Default Folder Locations 14
User-Supplied Consumables 15
Start the MiSeq
1 Switch the toggle power switch on the back of the instrument to the | (on)position.
NOTE
For best performance, leave the instrument on continuously. However, if the instrument must be turned
off, see
Shut Down the Instrument
on page 39. Wait a
minimum
of 60seconds before turning the power
switch back to the ON position
Figure 9 Power Switch Location
2 Wait for the system to load, and then log on to the operating system. If necessary, consult your facility
administrator for the user name and password.
When the operating system is loaded, the MiSeq Control Software (MCS) launches and initializes the
system automatically.
Customize System Settings
1 From the Home screen, select Run Options.
2 Select the Run Settings tab.
3 Select Post Run Wash or Maintenance Wash.
An instrument wash is required after each run. The software requires that a wash is performed before
setting up a subsequent run. The Post-Run Wash Option specifies the type of wash that is performed by
default. A post-run wash takes approximately 30 minutes. A maintenance wash takes approximately 1
hour.
4 Enter the address of the BaseSpace Onsite server location.
The Onsite Server setting is required if you use BaseSpace Onsite.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
13
5 Select Send Instrument Performance Data to Illumina to aid technical support to enable the Illumina
Proactive monitoring service. The name of the setting in the software interface might be different from the
name in this guide, depending on the version of MCS in use.
With this setting turned on, instrument performance data are sent to Illumina. This data helps Illumina
troubleshoot more easily and detect potential failures, enabling proactive maintenance and maximizing
instrument uptime. For more information on the benefits of this service, see
Illumina Proactive Technical
Note (document # 1000000052503)
.
This service:
u Does not send sequencing data.
u Requires that the instrument be connected to a network with internet access.
u Is turned on by default. To opt out of this service, disable the Send Instrument Performance Data to
Illumina to aid technical support setting.
6 For MiSeq Reporter, select or clear When using BaseSpace or BaseSpace Onsite, replicate analysis
locally on MiSeq.
The Replicate Analysis Locally setting specifies analysis processing locations when using BaseSpace or
BaseSpace Onsite. The setting provides the option to perform analysis both locally on the instrument and
in BaseSpace or BaseSpace Onsite.
If you select this option when using BaseSpace or BaseSpace Onsite, MiSeq Reporter launches
automatically after the run and performs analysis locally.
If you do not select this option when using BaseSpace or BaseSpace Onsite, MiSeq Reporter does not
launch automatically after the run and analysis is performed in BaseSpace or BaseSpace Onsite only.
If performing the VeriSeq PGS workflow with BlueFuse Multi, select this option.
Configure Notifications of BaseSpace Updates
1 From the Home screen, select Manage Instrument.
2 Select Software Update.
3 Select Automatically check for new software updates on BaseSpace.
Set Email Preferences
MiSeq can be configured to send an email notification when RTA analysis is complete, when on-instrument
secondary analysis is complete, or if a critical MiSeq software error occurs.
1 From the Home screen, select Run Options.
2 Select the Email Notifications tab.
3 Enter the following information:
u Local SMTP email server address—Use the on-screen keyboard to enter the local SMTP email server
address. If necessary, contact the facility administrator for this information.
u Sender address—Use the on-screen keyboard to enter the sender email address. This address can
be your email address or a different address specified for sending email notifications.
u Recipient addresses—Use the on-screen keyboard to enter the email addresses of each recipient to
receive notifications. Separate each email address with a comma. Select Test to send a test email to
notification recipients.
u Notify via email when—Select the checkbox for each of the run events that trigger a notification.
Set Default Folder Locations
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
14
MiSeq System Guide

Folders can be on a local network or on the instrument computer.
1 From the Home screen, select Run Options.
2 Select the Folder Settings tab.
3 Enter default locations for the following folders.
u Recipe Folder—Sets the default location for recipes. Recipes are XML files that the software uses to
perform the sequencing run. A recipe is created at the start of the run based on parameters in the
sample sheet, and then the recipe is copied to the output folder.
u Sample Sheet Folder—Sets the default location for sample sheets. Sample sheets are created before
library preparation and contain parameters for the run.
u Manifest Folder—Manifest files are required for some library types. See the sample prep
documentation for your sample prep kit, as well as the
Sample Sheet Quick Reference Guide
(document # 15028392)
.
u MiSeqOutput—For MiSeq Reporter, sets the default location for analysis output files. Change the
default output folder to a network location for sharing, long-term storage, and optionally using MiSeq
Reporter off-line. For more information, see
Run Folders
on page 1.
User-Supplied Consumables
Make sure that the following user-supplied consumables are available before beginning a run.
Consumable Supplier Purpose
Stock 1.0 N NaOH,
molecular biology-grade
General lab supplier Denaturing sample libraries and PhiX control DNA
Alcohol wipes, 70%
Isopropyl
or
Ethanol, 70%
VWR, catalog#95041-
714*
General lab supplier
Cleaning the flow cell holder
Disposable gloves,
powder-free
General lab supplier General use
Lab tissue, low-lint VWR, catalog#21905-
026*
Cleaning the flow cell stage and the foil seal covering the load
samples reservoir
Lens paper, 4x6in. VWR, catalog#52846-
001*
Cleaning the flow cell
Microcentrifuge tubes General lab supplier Denaturing and diluting sample libraries and PhiX control DNA
MiSeq tubes Illumina,
part # MS-102-9999
Washing the template line, for use with the VeriSeq PGS
workflow (optional for other workflows)
NaOCl, 5% Sigma-Aldrich, catalog
# 239305*
Washing the template line, for use with the VeriSeq
PGSworkflow (optional for other workflows)
Tween 20 Sigma-Aldrich, catalog
# P7949
Washing the instrument
Tweezers, square-tip
plastic (optional)
McMaster-Carr, catalog
# 7003A22*
Removing flow cell from flow cell shipping container
Water, laboratory-grade General lab supplier Washing the instrument
* or laboratory-grade equivalent
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
15
MiSeq System Guide
Guidelines for Laboratory-Grade Water
Always use laboratory-grade water or deionized water to perform instrument procedures. Never use tap
water. Use only the following grades of water or equivalents:
u Deionized water
u Illumina PW1
u 18Megohms (MΩ) water
u Milli-Q water
u Super-Q water
u Molecular biology grade water
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
16
MiSeq System Guide

Chapter 3 Sequencing
Introduction 17
Run Duration 17
MiSeq Workflow 18
Thaw Reagent Cartridge 19
Inspect the Reagent Cartridge 19
Denature and Dilute Libraries 20
Load Sample Libraries 20
Set Up a Run Using MCS 20
Clean the Flow Cell 21
Load the Flow Cell 22
Load Reagents 23
Starting the Run 25
Monitor the Run 26
Perform a Post-Run Wash 28
Introduction
To perform a sequencing run on the MiSeq, follow the setup steps described in this chapter.
After the run begins, no other user intervention is required.
The sequencing run can be monitored from the Sequencing screen or remotely using the Sequencing
Analysis Viewer (SAV). This optional application is available for download from the Illumina website.
After the sequencing run is complete, perform an instrument wash.
Run Duration
Run duration is based on the number of cycles performed. You can perform a paired-end run up to 2 x 301
sequencing cycles plus any Index Reads with MCS v2.3 or later.
Additionally, run duration is based on the version of MiSeq reagents you are using and any performance
enhancing upgrades installed on your instrument.
For expected durations and other specifications, visit the MiSeq System specifications page on the Illumina
website.
Number of Cycles in a Read
In a sequencing run, the number of cycles performed in a read is one more cycle than the number of cycles
analyzed. The extra cycle is required for phasing and prephasing calculations.
For example, a paired-end 300-cycle run performs two reads of 301 cycles (2 x 301) for a total of 602 cycles.
At the end of the run, 2 x 300 cycles are analyzed.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
17

MiSeq Workflow
Prepare the prefilled reagent cartridge for use.
Denature and dilute libraries (does not apply to all library types). See
Preparing
Libraries for Sequencing on the MiSeq (document # 15039740)
.
Load the library mix onto the reagent cartridge in the designated reservoir.
From the software interface, select Sequence to start the run setup steps.
[Optional] Connect to BaseSpace or BaseSpace Onsite.
Wash and thoroughly dry the flow cell.
Load the flow cell.
Load the PR2 bottle and make sure that the waste bottle is empty.
Load the reagent cartridge.
Review run parameters and pre-run check results.
Select Start Run.
Monitor your run from the MCS interface or from another computer using
Sequencing Analysis Viewer (SAV).
Perform a post-run wash.
Cluster Generation
During cluster generation, single DNA molecules are bound to the surface of the flow cell, and then bridge-
amplified to form clusters.
Sequencing
Following cluster generation, clusters are imaged using LED and filter combinations specific to each of the
four fluorescently labeled dideoxynucleotides. After imaging of a tile is complete, the flow cell is moved into
place to expose the next tile. The process is repeated for each cycle of sequencing. Following image analysis,
the software performs base calling, filtering, and quality scoring.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
18
MiSeq System Guide

Analysis
When the run is complete, MiSeq Reporter analysis software launches automatically to perform secondary
analysis, which includes alignment and variant calling. You can monitor secondary analysis using an internet
connection from another computer. For more information, see the
MiSeq Reporter Software
on page 8
Thaw Reagent Cartridge
Thaw the reagent cartridge using a room temperature water bath.
NOTE
Alternatively, thaw reagents overnight in 2°C to 8°C storage. Reagents are stable up to one week when
stored at this temperature.
1 Remove thereagent cartridge from -25°C to -15°C storage.
2 Place the reagent cartridge in a water bath containing enough room temperature deionized water to
submerge the base of the reagent cartridge. Do not allow the water to exceed the maximum water line
printed on the reagent cartridge.
Figure 10 Maximum Water Line
3 Allow the reagent cartridge to thaw in the room temperature water bath until it is thawed completely.
u MiSeq v3 cartridges— ~ 60-90 minutes.
u MiSeq v2 cartridges— ~ 60 minutes.
4 Remove the cartridge from the water bath and gently tap it on the bench to dislodge water from the base
of the cartridge. Dry the base of the cartridge.
Inspect the Reagent Cartridge
1 Invert the reagent cartridge ten times to mix the thawed reagents, and then inspect that all positions are
thawed.
2 Inspect the reagents in positions 1, 2, and 4 to make sure that they are fully mixed and free of
precipitates.
3 Gently tap the cartridge on the bench to reduce air bubbles in the reagents.
NOTE
The MiSeq sipper tubes go to the bottom of each reservoir to aspirate the reagents, so it is important
that the reservoirs are free of air bubbles.
4 Place the reagent cartridge on ice for up to six hours, or set aside at 2°C to 8°C until ready to set up the
run. For best results, proceed directly to loading the sample and setting up the run.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
19
MiSeq System Guide

WARNING
This set of reagents contains potentially hazardous chemicals. Personal injury can occur through inhalation,
ingestion, skin contact, and eye contact. Wear protective equipment, including eye protection, gloves, and
laboratory coat appropriate for risk of exposure. Handle used reagents as chemical waste and discard in
accordance with applicable regional, national, and local laws and regulations. For additional environmental,
health, and safety information, see the SDS at support.illumina.com/sds.html.
Denature and Dilute Libraries
If necessary for your library type, denature and dilute libraries, and add optional PhiX control. See
MiSeq
System Denature and Dilute Libraries Guide (document # 15039740)
. If you are performing the VeriSeq
PGSworkflow, see the
VeriSeq PGS Library Preparation Guide (document # 15052877)
.
This step does not apply to all library types
. Some Illumina sample preparation methods result in a ready-to-
use normalized concentration of pooled libraries. Refer to the sample preparation guide for the kit used to
prepare sample libraries.
NOTE
If you are using custom primers, prepare primers and set up the sample sheet as described in
MiSeq
Custom Primers Guide (document # 15041638)
.
Load Sample Libraries
When the reagent cartridge is fully thawed and ready for use, load prepared libraries onto the cartridge.
1 Clean the foil seal covering the reservoir labeled Load Samples with a low-lint lab tissue.
2 Pierce the foil seal with a clean 1 ml pipette.
3 Pipette 600 µl of prepared libraries into the reservoir Load Samples. Avoid touching the foil seal.
Figure 11 Load Libraries
`
4 Proceed directly to the run setup steps using the MiSeq Control Software (MCS) interface.
Set Up a Run Using MCS
1 From the Home screen, select Manage Instrument.
2 Select Reboot to reboot the system software.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
20
MiSeq System Guide

3 [Optional] From the Run Options screen, check the folder locations for MiSeqOutput, recipes, sample
sheets, and manifests. For more information, see
Set Default Folder Locations
on page 14.
4 From the Home screen, select Sequence to begin the run setup steps.
When you select Sequence on the Home screen, a series of run setup screens open in the following
order: BaseSpace Option, Load Flow Cell, Load Reagents, Review, and Pre-Run Check.
Set BaseSpace or BaseSpace Onsite Option
Optionally run secondary analysis using BaseSpace or BaseSpace Onsite.
1 From the BaseSpace Options screen, select or clear the Use BaseSpace for storage and analysis and
Use BaseSpace Onsite for storage and analysis checkboxes.
2 Select Next.
Clean the Flow Cell
1 Put on a new pair of powder-free gloves.
2 Using plastic forceps, grip the flow cell by the base of the plastic cartridge and remove it from the flow cell
container.
Figure 12 Remove Flow Cell
3 Lightly rinse the flow cell with laboratory-grade water until both the glass and plastic cartridge are
thoroughly rinsed of excess salts.
Excess salts can affect flow cell seating on the instrument. If salts dry in the imaging area, imaging can
also be affected.
Figure 13 Rinse Flow Cell
4 Using care around the black flow cell port gasket, thoroughly dry the flow cell and cartridge with a lint-
free lens cleaning tissue. Gently pat dry in the area of the gasket and adjacent glass.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
21
MiSeq System Guide

Figure 14 Flow Cell Ports and Gasket
5 Clean the flow cell glass with an alcohol wipe. Make sure that the glass is free of streaks, fingerprints, and
lint or tissue fibers.
NOTE
Do not use the alcohol wipe on the flow cell port gasket.
Figure 15 Dry Flow Cell
6 Dry excess alcohol with a lint-free lens cleaning tissue.
7 Make sure that the flow cell ports are free of obstructions and that the gasket is well-seated around the
flow cell ports.
If the gasket appears to be dislodged, gently press it back into place until it sits securely around the flow
cell ports.
Load the Flow Cell
1 Raise the flow cell compartment door, and then press the release button to the right of the flow cell
clamp.
The flow cell clamp opens.
Figure 16 Open Flow Cell Clamp
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
22
MiSeq System Guide

2 Make sure that the flow cell stage is free of lint. If lint or other debris is present, clean the flow cell stage
using an alcohol wipe or a lint-free tissue moistened with ethanol or isopropanol. Carefully wipe the
surface of the flow cell stage until it is clean and dry.
3 Holding the flow cell by the edges, place it on the flow cell stage.
Figure 17 Place Flow Cell on Stage
4 Gently press down on the flow cell clamp to close it over the flow cell.
As the flow cell clamp closes, alignment pins position the flow cell. An audible click indicates that the flow
cell clamp is secure.
Figure 18 Close Flow Cell Clamp
5 If the software does not identify the flow cell RFID, see
Resolve RFID Read Failure
on page 44.
6 Close the flow cell compartment door.
7 Select Next.
Load Reagents
Load PR2 and Check the Waste Bottle
1 Remove the bottle of PR2 from 2° to 8°C storage. Invert to mix, and then remove the lid.
2 Open the reagent compartment door.
3 Raise the sipper handle until it locks into place.
4 Remove the wash bottle and load the PR2 bottle.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
23
MiSeq System Guide

Figure 19 Load the PR2 Bottle
5 Empty the contents of the waste bottle into the appropriate waste container.
6 Slowly lower the sipper handle. Make sure that the sippers lower into the PR2 and waste bottles.
Figure 20 Lower Sipper Handle
7 If the software does not identify the RFID of the PR2 bottle, see
Resolve RFID Read Failure
on page 44.
8 Select Next.
Load the Reagent Cartridge
1 Open the reagent chiller door.
NOTE
Do not leave the reagent chiller door open for extended periods of time.
2 Hold the reagent cartridge on the end with the Illumina label, and slide the reagent cartridge into the
reagent chiller until the cartridge stops.
Always use the reagent cartridge associated with the type of flow cell that you loaded. If the reagent
cartridge is not compatible, a message appears on the screen. Select Back to load the appropriate
reagent cartridge or Home to return to the Home screen.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
24
MiSeq System Guide

Figure 21 Load Reagent Cartridge
3 Close the reagent chiller door.
4 If the software does not identify the RFID of the reagent cartridge, see
Resolve RFID Read Failure
on
page 44.
5 If the reagent cartridge is not compatible with the flow cell, a message appears. Select Back to load a
compatible cartridge, or select Exit to return to the Home screen.
6 Close the reagent compartment door.
7 Select Next.
Change Sample Sheet
Use the Change Sample Sheet command for the following:
u To select a sample sheet with a name that does not match the reagent cartridge barcode number
u When the software prompts you to choose a different sample sheet on the Review screen
Every run must have a sample sheet. By default, the software looks for a sample sheet file with a name
matching the barcode number of the reagent cartridge loaded on the instrument. If a sample sheet is not
found, a message appears that prompts you to browse to the location of the correct sample sheet for your
run.
To prevent the software from searching unsuccessfully, use the Change Sample Sheet command on the
Load Reagents screen to direct the software to the appropriate sample sheet.
1 Select Change Sample Sheet on the Load Reagents screen.
2 Select Browse to navigate to the sample sheet.
3 Select Open.
4 Select Save and Continue.
5 Select Next.
Starting the Run
After loading the flow cell and reagents, review the run parameters and perform a pre-run check before
starting the run.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
25
MiSeq System Guide

Review Run Parameters
1 Review Experiment Name, Analysis Workflow,and Read Length. These parameters are specified in the
sample sheet.
2 Select Change Folder to review the folder locations.
3 Modify as needed and then select Save.
4 Select Next.
Change Folders
To change folder locations, select Change Folder and browse to a preferred location. Using this option from
the Review screen changes folder locations for the current run only.
Review Pre-Run Check
The system performs a check of all run components, disk space, and network connections before starting
the run.
If any items do not pass the pre-run check, a message appears on the screen with instructions on how to
correct the error. For more information, see
Resolve Run Setup Errors
on page 44.
When all items successfully pass the pre-run check, select Start Run.
Important Notes Before Startingthe Run
WARNING
The MiSeq is sensitive to vibration. Touching the instrument after starting a run could adversely affect
sequencing results.
After selecting Start Run, do not open the flow cell compartment or the reagent compartment doors, or
touch the instrument monitor except to pause the run. For more information, see
Pause a Run
on page 42.
WARNING
Make sure to close all files on the MiSeq before starting a run, and do not open files during a run.
Monitor the Run
1 During the run, monitor run progress, intensities, and quality scores that appear on the Sequencing
screen. The Sequencing screen is view-only.
To monitor the run in greater detail, use the Sequencing Analysis Viewer (SAV) installed on a computer
independent of the instrument computer. A network connection is required.
Alternatively, if you are connected to BaseSpace, the run can be monitored using SAV in BaseSpace.
u Run Progress—Shows run progress in a status bar and lists the number of cycles completed.
u Intensity—Shows the value of cluster intensities of the 90
th
percentile for each tile.
The graphic in the Intensity area represents the number of tiles and number of surfaces being imaged:
u If the flow cell is imaged on the top surface only, a single-column graphic appears.
u If the flow cell is image on the top surface and bottom surface, a 2 column graphic appears.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
26
MiSeq System Guide
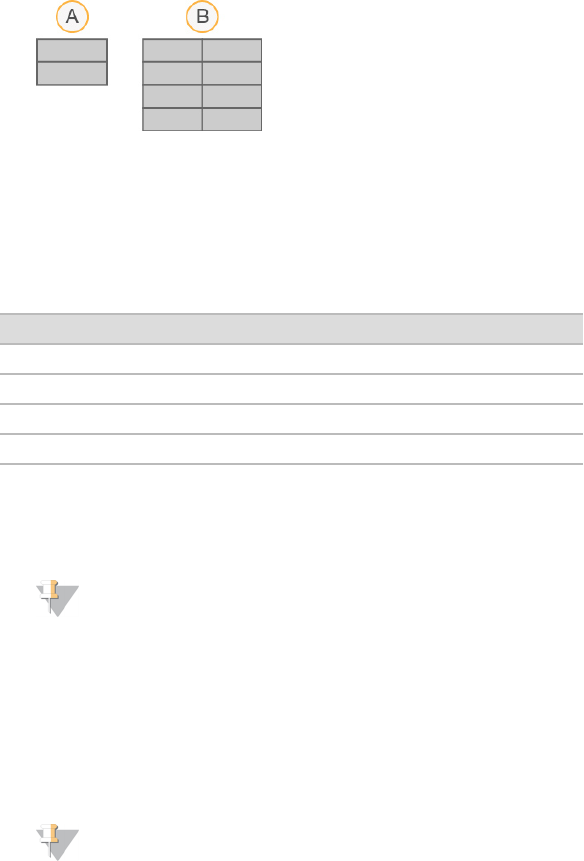
A Indicates 2 tiles, top surface only
B Indicates 4 tiles, top and bottom surface
u Q-Score All Cycles—Shows the average percentage of bases greater than Q30, which is a quality
score (Q-score) measurement. A Q-score is a prediction of the probability of a wrong base call. Q-
scores are calculated after cycle 25.
Q-Score Probability of Wrong Base Call
Q40 1 in 10,000
Q30 1 in 1,000
Q20 1 in 100
Q10 1 in 10
u Cluster Density (K/mm²)—Shows the number of clusters per square millimeter for the run.
u Clusters Passing Filter (%)—Shows the percentage of clusters passing filter based on the Illumina
chastity filter, which measures quality. This data appears only after cycle25.
NOTE
The chastity of a base call is the ratio of the intensity of the greatest signal divided by the sum of the 2
greatest signals. If more than 1 base call has a chastity value of less than 0.6 in the first 25 cycles, reads
do not pass the quality filter.
u Estimated Yield (Mb)—Shows the projected number of bases called for the run, measured in
megabases. This data appears only after cycle25.
2 When the run is complete, the Next button appears. Review the results on the Sequencing screen before
proceeding.
NOTE
The Sequencing screen remains viewable until Next is selected. After you select Next, it is not possible to
return to the Sequencing screen.
3 Select Next to exit the Sequencing screen and proceed to a post-run wash.
Template Generation
Template generation is the process where cluster positions over the entire flow cell surface are defined
according to X and Y coordinate position. Real-time analysis (RTA) uses early cycles of the run for template
generation.
After the template of cluster positions is generated, images produced over every subsequent cycle of imaging
are aligned against the template. Individual cluster intensities in all four nucleotide color channels are
extracted and base calls are produced from the normalized cluster intensities.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
27
MiSeq System Guide

Run Metrics
Run metrics appear on the Sequencing screen at different points in a run. During cluster generation steps, no
metrics appear.
After sequencing begins, the following metrics appear at the indicated cycles:
Metric Kit Cycle
Intensity MiSeq Reagent Kits, v3
MiSeq Reagent Kits, v2
MiSeq Reagent Kits, v1
Cycle 1–7
Cycle 1–4
Cycle 1–4
Intensity and Cluster Density MiSeq Reagent Kits, v3
MiSeq Reagent Kits, v2
MiSeq Reagent Kits, v1
Cycle 8–25
Cycle 5–25
Cycle 5–25
Intensity, Cluster Density, % PF,
Yield, and Q-scores
MiSeq Reagent Kits, v3
MiSeq Reagent Kits, v2
MiSeq Reagent Kits, v1
Cycle 26 through run completion
For MiSeq run specifications, visit the MiSeq System specifications page on the Illumina website
(www.illumina.com/systems/miseq/performance_specifications.ilmn).
RTA Analysis Results
The RTA analysis output from a sequencing run is a set of quality-scored base call files (*.bcl), which are
generated from the raw image files. For a list of RTA files and folders, see
RTA Folders and Files
on page 51.
Perform a Post-Run Wash
The post-run wash is the standard instrument wash performed between sequencing runs. Always perform an
instrument wash after completing a run. Follow the software prompts to load the wash components and
perform the wash. The post-run wash takes approximately 20 minutes.
Start the wash directly following the completion of a run. An instrument wash is required before you can set
up a subsequent run. To perform a post-run wash at a time other than directly following a run, use the
command on the Perform Wash screen to initiate the wash.
NOTE
Leave the used flow cell on the instrument. A flow cell must be loaded on the instrument to perform an
instrument wash.
Regular instrument washes ensure continued performance in the following ways:
u Flushes any remaining reagents from the fluidics lines and sippers
u Prevents salt accumulation and crystallization in the fluidics lines and sippers
u Prevents cross-contamination from the previous run
If you are using MCS v2.5 or later, you have the option to perform a post-run wash that includes a template
line wash with sodium hypochlorite solution (NaOCl). The wash takes approximately 30 minutes. See
Procedure with Template Line Wash
on page 30.
NOTE
If you are using the VeriSeq PGSworkflow, perform post-run washes that include a template wash. See
Procedure with Template Line Wash
on page 30.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
28
MiSeq System Guide

Consumables
u Tween 20
u Laboratory-grade water
u NaOCl (use with a post-run wash that includes a template line wash)
u MiSeq tube (part # MS-102-9999) (for post-run washes that include a template line wash)
Procedure
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 Prepare the wash components with fresh wash solution:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
3 When the run is complete, select Start Wash.
The software automatically raises the sippers in the reagent chiller.
Do not
select Perform optional template line wash on the Post-Run wash screen. The template line wash
requires a different procedure. See
Procedure with Template Line Wash
on page 30.
4 Open the reagent compartment door and reagent chiller door, and slide the used reagent cartridge from
the chiller.
5 Slide the wash tray into the reagent chiller until it stops, and then close the reagent chiller door.
6 Raise the sipper handle in front of the PR2 bottle and waste bottle until it locks into place.
7 Remove the PR2 bottle and replace it with the wash bottle.
NOTE
Discard the PR2 bottle after each run. Do not reuse any remaining PR2.
8 Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the reagent
compartment.
WARNING
This set of reagents contains potentially hazardous chemicals. Personal injury can occur through
inhalation, ingestion, skin contact, and eye contact. Wear protective equipment, including eye
protection, gloves, and laboratory coat appropriate for risk of exposure. Handle used reagents as
chemical waste and discard in accordance with applicable regional, national, and local laws and
regulations. For additional environmental, health, and safety information, see the SDS at
support.illumina.com/sds.html.
9 Slowly lower the sipper handle, making sure that the sippers lower into the wash bottle and waste bottle.
10 Close the reagent compartment door.
11 Select Next.
When the wash is complete, leave the used flow cell, wash tray, and wash bottle containing the remaining
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
29
MiSeq System Guide

wash solution on the instrument.
NOTE
The sippers remain in the down position, which is normal. Leave the unused wash solution in the wash tray
and wash bottle to prevent the sippers from drying out and air from entering the system.
Procedure with Template Line Wash
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 Prepare fresh NaOCl wash solution with laboratory-grade water:
a Add 36 µl of 5% NaOCl to 864 µl laboratory-grade water. These volumes result in a 1:25 NaOCl
dilution.
b Add 50 µl of the 1:25 NaOCl dilution to 950 µl of laboratory-grade water in a MiSeq tube (part # MS-
102-9999).
NOTE
Using the correct concentration of NaOCl is important. Make sure to check the percentage of NaOCl on
the product label. If the concentration is too high, it can make cluster generation fail in subsequent runs.
If 5% NaOCl is not available, make a 1 ml solution of 0.01% NaOCl in laboratory-grade water.
Do not
use
NaOCl with a maintenance wash or a standby wash
3 Prepare the wash components with fresh wash solution:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
4 Insert the MiSeq tube containing 0.01% NaOCl wash solution into position 17 of the wash tray until the
neck of the tube is flush with the tray. The tube displaces the Tween 20 and laboratory-grade water
wash solution from position 17.
Figure 22 MiSeq Tube in Position 17 of the Wash Tray
NOTE
Make sure to insert the MiSeq tube with NaOCl into tray position 17 only. Inserting the tube in another
position can make cluster generation fail in subsequent runs, and can damage the fluidic system of the
MiSeq instrument.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
30
MiSeq System Guide

5 When the run is complete, select Start Wash. The software automatically raises the sippers in the reagent
chiller.
6 Select Perform optional template line wash on the Post-Run Wash screen.
When using the VeriSeq PGS workflow, the option Perform optional template line wash is pre-selected for
you. The MCS tracks the type of post-run wash performed after each run. If Perform optional template
line wash is not selected for the post-run wash, a message on the Run Review screen reminds you the
next time you start a sequencing run.
7 Open the reagent compartment door and reagent chiller door, and slide the used reagent cartridge from
the chiller.
8 Slide the wash tray into the reagent chiller until it stops, and then close the reagent chiller door.
9 Raise the sipper handle in front of the PR2 bottle and waste bottle until it locks into place.
10 Remove the PR2 bottle and replace it with the wash bottle.
NOTE
Discard the PR2 bottle after each run. Do not reuse any remaining PR2.
11 Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the reagent
compartment.
WARNING
This set of reagents contains potentially hazardous chemicals. Personal injury can occur through inhalation,
ingestion, skin contact, and eye contact. Wear protective equipment, including eye protection, gloves, and
laboratory coat appropriate for risk of exposure. Handle used reagents as chemical waste and discard in
accordance with applicable regional, national, and local laws and regulations. For additional environmental,
health, and safety information, see the SDS at support.illumina.com/sds.html.
12 Slowly lower the sipper handle, making sure that the sippers lower into the wash bottle and waste bottle.
13 Close the reagent compartment door.
14 Select Next.
When the wash is complete, leave the used flow cell, wash tray, and wash bottle containing the remaining
wash solution on the instrument.
NOTE
The sippers remain in the down position. Leave the unused wash solution in the wash tray and wash bottle
to prevent the sippers from drying out and air from entering the system.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
31
MiSeq System Guide
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
32
MiSeq System Guide

Chapter 4 Maintenance
Maintenance Frequency 33
Maintenance Frequency for the VeriSeq PGS Workflow 33
Perform a Maintenance Wash 34
Perform a Standby Wash 36
Manage Files 38
Software Updates 39
Shut Down the Instrument 39
Maintenance Frequency
Perform the following maintenance procedures at the recommended intervals.
NOTE
If performing the VeriSeq PGS workflow, make sure to follow maintenance frequency guidelines for VeriSeq
PGS. See
Maintenance Frequency for the VeriSeq PGS Workflow
on page 33.
Activity Frequency
Post-Run Wash After every run
Maintenance Wash Monthly
Standby Wash To prepare for idle mode (if unused for ≥ 7 days), and every 30 days the instrument
remains idle
Instrument Shutdown As needed
Table 2 Maintenance During Normal Operation
Activity Frequency
Standby Wash Monthly
Instrument Shutdown As needed
Table 3 Maintenance During Idle Mode (≥ 7 days unused)
Maintenance Frequency for the VeriSeq PGS Workflow
If performing the VeriSeq PGSworkflow, perform the following maintenance procedures at the recommended
intervals.
Activity Frequency
Post-Run Wash After every run
Maintenance Wash Monthly
Post-Run Wash from the Perform Wash Screen After in idle mode (unused for > 3 days)
Standby Wash To prepare for idle mode (if unused for ≥ 7 days), and
every 30 days the instrument remains idle
Instrument Shutdown As needed
Table 4 Maintenance During Normal Operation
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
33

Activity Frequency
Standby Wash Monthly
Instrument Shutdown As needed
Table 5 Maintenance During Idle Mode (≥ 7 days unused)
Perform a Maintenance Wash
Perform a maintenance wash every 30 days to ensure optimal performance.
The maintenance wash takes approximately 90 minutes to complete. The wash includes a series of three
wash steps that thoroughly flush the system.
You can configure your instrument to perform a maintenance wash between runs. For more information,
Customize System Settings
on page 13.
User-Supplied Consumables
u Tween 20 (Sigma-Aldrich, catalog # P7949)
u Laboratory-grade water
Procedure
1 Make sure that a used flow cell is loaded on the instrument.
2 From the Home screen, select Perform Wash.
3 From the Perform Wash screen, select Perform Maintenance Wash.
The software automatically raises the sippers in the reagent chiller.
Perform First Wash
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 Prepare the wash components with fresh wash solution:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
3 Load the wash tray and wash bottle onto the instrument:
a Open the reagent compartment door and reagent chiller door, and slide the used reagent cartridge
or wash tray from the chiller.
b Slide the wash tray into the reagent chiller until it stops. Close the reagent chiller door.
c Raise the sipper handle in front of the PR2 bottle and waste bottle until it locks into place, and
replace the PR2 bottle with the wash bottle.
NOTE
Discard the PR2 bottle after each run. Do not reuse any remaining PR2.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
34
MiSeq System Guide
a Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the
reagent compartment.
b Slowly lower the sipper handle, making sure that the sippers lower into the wash bottle and waste
bottle.
c Close the reagent compartment door.
4 Select Next. The first wash begins.
Perform Second Wash
Always use fresh wash solution for each wash step. Reusing wash solution from the previous wash can return
waste to the fluidics lines.
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water, as follows:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 When the first wash is complete, remove the wash tray and wash bottle, and discard the remaining wash
solution.
3 Refill the wash components with fresh wash solution, as follows:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
4 Load the wash tray and wash bottle, as follows:
a Slide the wash tray into the reagent chiller until it stops. Close the reagent chiller door.
b Load the wash bottle and slowly lower the sipper handle, making sure that the sippers lower into the
wash bottle and waste bottle.
c Close the reagent compartment door.
5 Select Next. The second wash begins.
Perform Final Wash
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 When the second wash is complete, remove the wash tray and wash bottle, and discard the remaining
wash solution.
3 Refill the wash components with fresh wash solution:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
4 Load the wash tray and wash bottle:
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
35
MiSeq System Guide

a Slide the wash tray into the reagent chiller until it stops. Close the reagent chiller door.
b Load the wash bottle and slowly lower the sipper handle, making sure that the sippers lower into the
wash bottle and waste bottle.
c Close the reagent compartment door.
5 Select Next. The final wash begins.
After the Wash
When the wash is complete, leave the used flow cell, wash tray, and wash bottle containing the remaining
wash solution on the instrument.
NOTE
The sippers remain in the down position, which is normal. Leave the unused wash solution in the wash tray
and wash bottle to prevent the sippers from drying out and air from entering the system.
Perform a Standby Wash
When there are no plans to use the instrument within the next seven days, prepare the instrument and
instrument fluidics lines to sit idle by performing a standby wash. Perform a standby wash every 30 days the
instrument sits idle.
The standby wash takes approximately two hours to complete. The wash performs two consecutive washes
that flush each position of any remaining reagents or salt accumulation. Each wash takes approximately 60
minutes.
When the standby wash is complete, the instrument is in standby mode and a message appears on the
Home screen stating the status of the instrument. When the instrument is in standby mode, a maintenance
wash must be performed before a sequencing run can be initiated.
User-Supplied Consumables
u Tween 20 (Sigma-Aldrich, catalog # P7949)
u Laboratory-grade water
Procedure
1 Make sure that a used flow cell is loaded on the instrument.
2 From the Home screen, select Perform Wash.
3 From the Wash Options screen, select Perform Standby Wash.
The software automatically raises the sippers in the reagent chiller.
Perform First Wash
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 Prepare the wash components with fresh wash solution:
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
36
MiSeq System Guide

a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
3 Load the wash tray and wash bottle onto the instrument:
a Open the reagent compartment door and reagent chiller door, and slide the used reagent cartridge
or wash tray from the chiller.
b Slide the wash tray into the reagent chiller until it stops. Close the reagent chiller door.
c Raise the sipper handle in front of the PR2 bottle and waste bottle until it locks into place, and
replace the PR2 bottle with the wash bottle.
NOTE
Discard the PR2 bottle after each run. Do not reuse any remaining PR2.
a Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the
reagent compartment.
b Slowly lower the sipper handle, making sure that the sippers lower into the wash bottle and waste
bottle.
c Close the reagent compartment door.
4 Select Next. The first wash begins.
Perform Second Wash
Always use fresh wash solution for each wash step. Reusing wash solution from the previous wash can return
waste to the fluidics lines.
1 Prepare fresh wash solution with Tween 20 and laboratory-grade water, as follows:
a Add 5 ml 100% Tween 20 to 45 ml laboratory-grade water. These volumes result in 10% Tween 20.
b Add 25 ml 10% Tween 20 to 475 ml laboratory-grade water. These volumes result in a 0.5% Tween
20 wash solution.
c Invert five times to mix.
2 When the first wash is complete, remove the wash tray and wash bottle, and discard the remaining wash
solution.
3 Refill the wash components with fresh wash solution, as follows:
a Add 6 ml wash solution to each reservoir of the wash tray.
b Add 350 ml wash solution to the 500 ml wash bottle.
4 Load the wash tray and wash bottle, as follows:
a Slide the wash tray into the reagent chiller until it stops. Close the reagent chiller door.
b Load the wash bottle and slowly lower the sipper handle, making sure that the sippers lower into the
wash bottle and waste bottle.
c Close the reagent compartment door.
5 Select Next. The second wash begins.
After the Wash
When the wash is complete, leave the used flow cell, wash tray, and wash bottle containing the remaining
wash solution on the instrument.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
37
MiSeq System Guide

NOTE
The sippers remain in the down position, which is normal. Leave the unused wash solution in the wash tray
and wash bottle to prevent the sippers from drying out and air from entering the system.
Manage Files
Select the Manage Files on the Home screen to move, upload, delete files, or rename sample sheets on the
instrument computer.
Delete Files
1 From any tab on the Manage Files screen, select Browse to navigate to files accessible to the instrument.
2 Select from the following options:
u Select the checkbox next to individual files or folders in the list.
u Select the checkbox to the left of the Delete button to select all files and folders in the list. This option
is available for Runs, Sample Sheets, Manifests, Genomes, and Recipes.
3 Select Delete.
NOTE
The Delete command is available on all tabs except Bundle Logs.
Move Run Folders
The Move command
copies
the run folder to the new location and then
deletes
the folder from the old
location.
1 From the Runs tab on the Manage Files screen, select Browse to navigate to files accessible to the
instrument.
2 Select the checkbox next to individual files or folders in the list.
3 Select Move.
4 Select Browse Network and select a new location for the files or folders.
5 Select OK.
Upload Files
The Upload command is available for Sample Sheets, Manifests, Genomes, and Recipes. If the MiSeq is not
connected to a network, use this feature to upload files to the instrument computer from a USB drive.
1 From the tab on the Manage Files screen, select Browse to navigate to files accessible to the instrument.
2 Select Upload.
3 Select Browse Network and browse to the location on a USBdrive where the file resides.
4 Select OK.
The file is uploaded to the folder indicated in the Directory field.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
38
MiSeq System Guide
Rename Sample Sheets
1 From the Sample Sheets tab on the Manage Files screen, select from the following options:
u Select the checkbox next to individual sample sheets.
u Select the checkbox to the left of the Delete button to select all sample sheets in the list.
2 Select Rename.
3 Select the keyboard icon and use the on-screen keyboard to rename sample sheets.
4 Select Next.
5 Select Back.
Software Updates
If your system is connected to a network with internet access, you can automatically update the instrument
software from the Home screen. You can also configure the software to check automatically for BaseSpace
updates. For more information, see
Configure Notifications of BaseSpace Updates
on page 14.
If your instrument is not connected to a network with internet access, you can update the software manually.
Update Software Automatically
When software updates are available, the Update Available button appears on the Home screen. Otherwise,
this button is not visible. Make sure your MiSeq is connected to a network with internet access to enable this
option.
1 From the Home screen, select Update Available.
2 Confirm the command to update in the dialog box.
Reboot of the instrument is required. Installation of the update begins automatically upon reboot.
Update Software Manually
Use the Manual Update feature to update instrument control software and analysis software from the MiSeq
interface by browsing to the location of the installable software file.
1 From the Home screen, select Manage Instrument.
2 Select Software Update.
3 Select Browse to navigate to the location of the installable file for the new software version.
4 When the path to the installable software file appears on the screen, select Save and Update.
5 Confirm the command to update in the dialog box.
Reboot of the instrument is required. Installation of the update begins automatically upon reboot.
Shut Down the Instrument
It is best to leave the instrument on at all times. However, if the instrument must be turned off, use the
following procedure to shut down Windows and prepare the fluidics lines.
1 Perform a maintenance wash. For more information, see
Perform a Maintenance Wash
on page 34.
2 Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the reagent
compartment.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
39
MiSeq System Guide

3 Close the reagent compartment door.
4 From the Home screen, select Manage Instrument.
5 Select Shut Down.
This command shuts down the software.
6 Toggle the power switch to the OFF position.
NOTE
Any time that you turn off the instrument, wait a
minimum
of 60seconds before turning the power switch
back to the ON position.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
40
MiSeq System Guide

Appendix A Troubleshooting
Introduction 41
Bundle Logs for Troubleshooting 41
Perform a System Check 42
Pause or Stop a Run 42
Raise Reagent Cartridge Sippers Manually 44
Resolve Run Setup Errors 44
Resolve RFID Read Failure 44
Perform a Volume Test 45
Measure Expected Wash Volumes 46
Configure System Settings 46
Introduction
This section describes common troubleshooting steps to take before contacting Illumina Technical Support.
For most errors, an on-screen message appears with instructions for correcting the error.
For technical questions, visit the MiSeq support pages on the Illumina website for access to frequently asked
questions, or log in to your MyIllumina account for access to support bulletins.
For problems with run quality or performance, contact Illumina Technical Support. For more information, see
Technical Assistance
on page 57.
Illumina Technical Support representatives typically request copies of run-specific files for troubleshooting
purposes. You can use the Bundle Logs tab on the Manage Files screen to combine and zip the files required
for troubleshooting. See
Bundle Logs for Troubleshooting
on page 41.
Bundle Logs for Troubleshooting
Bundle Logs is a feature that bundles files to send to Illumina Technical Support for troubleshooting. Use the
Bundle Logs tab on the Manage Files screen to select a group of files, called a
bundle
. The bundle is zipped
automatically.
The Bundle Logs feature groups the files from a run into one bundle type at time. Repeat the Bundle Logs
procedure for each run and bundle type IlluminaTechnical Support requests.
1 On the Manage Files screen, select the Bundle Logs tab.
2 Select Browse to navigate to the location of the MiSeqOutput folder.
3 Click in the blue box next to the run, and in the blue circle next to the bundle type requested by Illumina
Technical Support.
4 Select Bundle Logs.
A Bundle Files screen opens with information about the bundle, including a list of individual files the
bundle contains.
For more information on the individual folders and files of the Bundle Logs feature, see
MiSeq Output and
Analysis Folders Quick Reference Card (document # 15034791)
.
5 Select Next.
6 Navigate to a location where you want the zipped bundle files saved.
7 Select Save.
When the files finish bundling, the Bundle Logs tab reopens.
8 Send the zipped bundle to Illumina Technical Support.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
41

Figure 23 Bundle Logs Tab
Perform a System Check
The System Check screen is typically used to connect with an Illumina Technical Support representative
during a Live Help session. Use of this feature is not required during normal operation or for instrument
maintenance.
Some system checks can be performed before contacting Illumina Technical Support, such as the Volume
Test. A volume test checks the health of the fluidics system by estimating the flow volume as bubbles pass by
the sensors. For more information, see
Perform a Volume Test
on page 45.
1 From the Home screen, select Manage Instrument.
2 Select System Check.
3 Do one of the following:
u Select the individual tests you want to perform.
u Choose Select All to perform all tests.
4 Select Next.
When complete, the test results appear on the screen.
5 [Optional] Select Show Details to see a summary of the results on the software interface.
6 [Optional] Select Export Results to export the results in a *.csv file format to a USB drive.
7 Select Done.
Pause or Stop a Run
The MiSeq is designed to complete a run from beginning to end without user intervention. However, it is
possible to pause a run or stop a run from the Sequencing screen.
Pause a Run
You can temporarily pause a run before it has completed. For example, a run can be paused if you suspect
that the waste bottle is
full. Paused runs can be resumed.
Document # 1000000061014 v00
For Research Use Only. Not for use in diagnostic procedures.
42
MiSeq System Guide

CAUTION
Do not pause a run during cluster generation or within the first eight cycles of sequencing. It is not possible to
resume a run that was paused during this time. See
Run Metrics
on page 28 for cycle information for MiSeq
reagent cartridge kits.
When you select Pause, the current command is completed before pausing the run and placing the flow cell
in a safe state.
To pause a run from the Sequencing screen, select Pause. The button changes to Resume. When you are
ready to resume the run, select Resume.
Figure 24 Sequence Screen of a Paused Run
Stop a Run
You can stop a run during sequencing before the run has completed using the Stop button on the
Sequencing screen. You might stop a run if the run was set up incorrectly, if the data quality is bad, or if you
experience a hardware error.
When a run is stopped, the current command is not completed and the flow cell stage moves to the forward
position. Real-time Analysis software continues analysis for the last completed cycle.
Figure 25 Stopping a Run
Stopping a run is final.
A stopped run cannot be resumed. The only option is to proceed to an instrument
wash.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
43
MiSeq System Guide

Raise Reagent Cartridge Sippers Manually
The reagent cartridge sippers might not raise automatically if a run was interrupted unexpectedly, or if an
error occurred during the run. To remove the reagent cartridge, raise the reagent cartridge sippers manually.
1 On the Home screen, select Perform Wash.
2 Select Raise Sippers.
3 Remove the reagent cartridge.
Resolve Run Setup Errors
If any checks in the pre-run check fail, a red icon appears next to the item. A message appears on the
screen that describes the error and how to correct it.
Error Action
Flow Rate Measured
The flow rate check screen opens. Use the drop-down list or on-screen keyboard to enter
the following:
• Solution: PR2
• Volume: 250
• Aspirate Rate: 2500
• Dispense Rate: 2500
Select Pump. If the error persists, set the volume to pump 500 µl PR2 and repeat the
process. When fluids have been pumped, select Restart Check.
When the pre-run check is successful, the Start Run button becomes active.
If the flow check fails again, reseat the flow cell to make sure that flow is not interrupted due
to misalignment. Inspect the flow cell gasket for lint or irregularities.
Free Disk Space
If disk space is low, a message appears indicating how much disk space is required. Use
the Manage Files feature to clear the required space from the instrument computer.
Network Connection Active
Make sure that the network cable is plugged into the instrument.
If the network connection is not restored, select Reboot on the Manage Instrument screen
to reboot the software.
If the connection is still not restored, select Shut Down on the Manage Instrument screen,
and then turn off the instrument using the power switch. Wait at least 60 seconds, and then
turn on the instrument and start the software.
Primary Analysis Ready
Primary analysis from the previous run is not complete. The default time to allow primary
analysis to complete is 1 hour, and a countdown appears on the screen. The options are to
wait 1 hour or select Terminate Analysis. Secondary analysis stops for any incomplete
cycles.
Sample Sheet Present
If you did not name your sample sheet with the reagent cartridge ID for your run, the
instrument cannot locate the appropriate sample sheet automatically. Browse to the sample
sheet for your run.
If you named your sample sheet with the reagent cartridge ID for your run, make sure that
the sample sheet is located in the default sample sheet folder. Check the default folder
location in Run Options on the Home screen.
Make sure that the sample sheet file extension is *.csv.
If the sample sheet is missing, create a sample sheet and copy it to the sample sheet
locations specified in Run Options.
When you have located a sample sheet, select Restart Check.
Resolve RFID Read Failure
If the system cannot read the RFID of a consumable, you can obtain a temporary bypass code from the
Illumina website. A temporary bypass code expires in seven days.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
44
MiSeq System Guide

1 Always retry the RFID read before proceeding. If the RFID fails a second time, select Get Code.
2 From a computer with internet access, go to my.illumina.com and select Sign In from the toolbar on the
top of the page.
3 Log in to your MyIllumina account.
Your name replaces the Sign In button on the toolbar.
4 Hover over your name and select Account. In the My Tools column, click MiSeq Self-Service.
5 On the MiSeq Self-Service page, enter the MiSeq serial number.
6 From the Type of Override Code drop-down list, select RFID Override.
7 To generate the code, select Get Code.
8 Return to the MCS interface and select Enter Code.
9 Enter the temporary bypass code using the on-screen keyboard, and then select Next.
10 Enter the barcode number of the flow cell, PR2 bottle, or reagent cartridge.
Consumable Barcode Number Location
Flow Cell Above the barcode on the flow cell container label.
Flow cell barcode numbers begin with an A (standard), G (micro), or D (nano). Example: A0E61
PR2 Bottle Below the barcode on the PR2 bottle label.
Example: MS0011881-PR2
Reagent Cartridge Below the barcode on the reagent cartridge label.
Example: MS0010744-300
11 If you are entering a bypass code for the reagent cartridge, enter the version number of the kit. Select
Enter Reagent Kit Barcode to enter the reagent cartridge barcode number and kit version number
manually.
CAUTION
Entering the incorrect reagent kit version can negatively affect sequencing data.
12 Select Enter.
Perform a Volume Test
An obstruction in the fluidics lines can cause poor reagent delivery and affect sequencing results. If an
obstruction in the fluidics lines is suspected, perform a volume test.
A volume test checks the health of the fluidics system by estimating the volume between bubbles as they
pass by the sensors. To perform a volume test, the wash tray and wash bottle must be loaded with
laboratory-grade water and a used flow cell must be in place. Follow the onscreen prompts to perform the
test.
1 Make sure that a used flow cell is loaded on the instrument.
2 From the Home screen, select Manage Instrument.
3 Select System Check.
4 Select Conduct Volume Test, and then select Next.
5 Fill each reservoir of the wash tray with 6 ml laboratory-grade water.
6 Fill the 500 ml wash bottle with 350 ml laboratory-grade water.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
45
MiSeq System Guide

7 Load the wash tray and wash bottle onto the instrument.
a Open the reagent compartment door and reagent chiller door, and slide the wash tray into the
reagent chiller until it stops. Close the reagent chiller door.
b Raise the sipper handle until it locks in place, and load the wash bottle.
c Remove the waste bottle and discard the contents appropriately. Return the waste bottle to the
reagent compartment.
d Slowly lower the sipper handle, making sure that the sippers lower into the wash bottle and waste
bottle.
8 Following the on-screen prompts, remove any droplets from the wash bottle sipper, as follows.
a Slowly raise the sipper handle and check the wash bottle sipper for the presence of a large water
droplet.
b Slowly lower the sipper handle far enough into the water to allow the surface tension to remove the
droplet.
c Slowly raise the sipper handle and check the wash bottle sipper for the presence of a large water
droplet.
d Slowly lower the sipper handle completely, making sure that the sippers lower into the wash bottle
and waste bottle.
9 Select Next.
When the volume test is complete, the results appear on the screen.
If the test did not pass, perform a maintenance wash. See
Perform a Maintenance Wash
on page 34.
10 When the maintenance wash is complete, repeat the volume test.
Measure Expected Wash Volumes
Measuring expected wash volumes confirms that wash fluidics are performing efficiently.
1 Before beginning a wash, empty the waste bottle.
2 When the wash is complete, measure the wash volume in the waste bottle.
Wash Type Expected Wash Volume
Post Run Wash 17.25 ml
Post Run Wash that includes a Template Line Wash 25.5 ml
Standby Wash 46 ml
Maintenance Wash 51.75 ml
Configure System Settings
The MCS includes several screens that access commands to configure the system. Typically software
settings are configured during MiSeq installation.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
46
MiSeq System Guide
Configure IP and DNS Settings
Configure IPaddress and DNS server addresses if required due to a network or facility change.
1 From the Home screen, select Manage Instrument.
2 Select System Settings.
u Select Obtain an IP address automatically or Use the following IP address.
If you select Use the following IP address, enter an IPaddress, subnet mask, and default gateway.
u Select Obtain DNS address automatically or Use the following DNS server addresses.
If you select Use the following DNS server addresses, enter a preferred and alternate DNS server
address.
3 Select Save and Continue.
Change System Credentials
Changing the system user name and password on the Systems Settings screen, also updates the credentials
for MiSeq Reporter and BaseSpace or BaseSpace Onsite.
1 From the Home screen, select Manage Instrument.
2 Select System Settings.
3 Select Save and Continue to progress to the third screen in the series of screens.
4 Select This account.
5 Enter the domain name (Domain\MiSeq1, for example) and password.
6 Select Save and Continue.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
47
MiSeq System Guide
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
48
MiSeq System Guide

Appendix B Output Files and Folders
Run Folders 49
MiSeqOutput Folder Contents 49
RTA Folders and Files 51
Run Folders
Each run on the MiSeq generates 3 run folders, each with a specific purpose:
u D:\Illumina\MiSeqTemp—When the run begins, a temporary run folder is written to the local drive of the
instrument computer and used as a working area for MCS and RTA. There is no need to access the
MiSeqTemp folder. Contents of this folder are deleted after 7 days.
u D:\Illumina\MiSeqOutput—RTA copies files from the MiSeqTemp folder to the MiSeqOutput folder. As
primary analysis files are generated, RTA copies files back to the MiSeqTemp folder and populates the
MiSeqAnalysis folder. Focus images and thumbnail images are not copied to the MiSeqAnalysis folder.
See
RTA Folders and Files
on page 51.
u You can change the location of the output folder in the Output Folder field on the Run Options screen. For
more information, see
Set Default Folder Locations
on page 14.
u D:\Illumina\MiSeqAnalysis—When analysis by RTA is complete, MiSeq Reporter accesses the
MiSeqAnalysis folder on the instrument local drive to begin secondary analysis. All files written to the
MiSeqAnalysis folder are copied back to the MiSeqOutput folder. For more information, see
MiSeqOutput
Folder Contents
on page 49.
If you are using BaseSpace for analysis without replicating analysis locally, the MiSeqAnalysis folder on the
instrument local drive is empty.
Root Folder Naming
The root run folder name identifies the date of the run, the instrument number, and the flow cell used for the
run.
By default, the folder name uses the following format:
YYMMDD_<InstrumentNumber>_<Run Number>_<FlowCellBarcode>
The run number increments by one each time a run is performed on a given instrument.
MiSeqOutput Folder Contents
After RTA completes analysis, the MiSeqOutput folder is populated with files necessary for secondary
analysis by MiSeq Reporter. When secondary analysis is complete, the MiSeqOutput and MiSeqAnalysis
folders are identical except that the MiSeqOutput folder contains 2 subfolders for images files: Images and
Thumbnail_Images. These subfolders are not required for secondary analysis.
Files
The files that are copied to the output and analysis folders include the following:
u SampleSheet.csv—Provides parameters for the run and subsequent analysis. At the start of the run, the
sample sheet is copied to the root folder and renamed SampleSheet.csv. Copies are written to
Data\Intensities and Data\Intensities\BaseCalls.
u runParameters.xml—Contains a summary of run parameters and information about run components,
such as the RFID of the flow cell and reagents associated with the run.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
49
u RunInfo.xml—Contains high-level run information, such as the number of reads and cycles in the
sequencing run, and whether a read is indexed.
Folders
The folders that are copied to the output and analysis folders include the following folders generated during
the sequencing run:
u <Run folder name>\Config—Contains configuration files for the run.
u <Run folder name>\Data—Contains subfolders Intensities, BaseCalls, and Alignment. Data generated
from MiSeq Reporter are located in the Alignment subfolder.
u <Run folder name>\Data\RTA Logs—Contains log files that describe each step performed by RTA for
each Read.
u <Run folder name>\Data\Intensities\BaseCalls—Contains subfolders with base call (*.bcl) files, matrix
files, and phasing files. MiSeq Reporter writes FASTQ files to this folder during secondary analysis. For
more information, see the
MiSeq Reporter Software Guide (document #15042295)
.
u <Run folder name>\Recipe—Contains the recipe used for the run.
u <Run folder name>\Logs—Contains log files that describe every step performed by the instrument for
each cycle.
u <Run folder name>\InterOp—Contains binary files used by Sequencing Analysis Viewer (SAV) to
summarize various primary analysis metrics such as cluster density, intensities, quality scores, and overall
run quality.
All other files and folders created in the temporary run folder are not copied to the output and analysis folders.
They contain temporary files that are not required for analysis or troubleshooting.
MiSeq Reporter adds other folders, such as the Alignment folder, during secondary analysis. For more
information, see the
MiSeq Reporter Software Guide (document #15042295)
.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
50
MiSeq System Guide

RTA Folders and Files
The following table describes the folders and files generated by real-time analysis (RTA) during primary
analysis. Many of these files are used for secondary analysis by the MiSeq Reporter software.
Key File Subfolder Description
RTAComplete.txt Root folder A marker file generated when base call analysis is complete.
The presence of this file triggers the start of secondary
analysis.
SampleSheet.csv Root folder This file is read and copied to the run folder before the run, and
later used for secondary analysis.
RunInfo.xml Root folder Identifies the boundaries of the reads (including index reads)
and the quality table selected for run.
*.bcl files Data\
Intensities\BaseCalls\
L001\
CX.X
Each *.bcl file contains RTA base calling and base quality
scoring results for 1 cycle, 1 tile.
*.stats files Data\
Intensities\BaseCalls\
L001\
CX.X
*.stats files contain RTA base calling statistics for a given
cycle/tile.
*.filter files Data\
Intensities\BaseCalls
*.filter files contain filter results per tile.
*.txt Data\RTALogs Log files from primary analysis.
*.cif files Data\
Intensities\L001\
CX.X
Each binary *.cif file contains RTA image analysis results for 1
cycle, 1 tile. For more information, see
Flow Cell Tile
Numbering
on page 52.
*.locs files Data\
Intensities\BaseCalls\
L001
Reports the cluster coordinates. Each *.locs file represents 1
tile.
*.jpg files Thumbnail_Images\
L001\
CX.X
Thumbnail images generated for each cycle and base, and
can be used to troubleshoot a run. These files are used for
image analysis and are not copied to the Analysis folder. See
Flow Cell Tile Numbering
on page 52 for image file names.
Flow Cell Tiles
During the sequencing run, the single lane of the flow cell is imaged in small imaging areas called tiles. All
MiSeq flow cells have a single lane, but the number of tiles differ depending on the type of flow cell you are
using.
Flow Cell MiSeq Reagent Kit Tiles Imaging Surface Total Tiles Imaged
Standard Flow Cell MiSeq Reagent Kits, v3 19 tiles Top and bottom 38 tiles total
PGS Flow Cell MiSeq Reagent Kit v3-PGS 19 tiles Top and bottom 38 tiles total
Standard Flow Cell MiSeq Reagent Kits, v2 14 tiles Top and bottom 28 tiles total
Micro Flow Cell MiSeq Reagent Micro Kits, v2 4 tiles Top and bottom 8 tiles total
Nano Flow Cell MiSeq Reagent Nano Kits, v2 2 tiles Top only 2 tiles total
When the tiles are imaged during the sequencing run, 1 output file is generated for each tile. For more
information, see
Flow Cell Tile Numbering
on page 52.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
51
MiSeq System Guide

Flow Cell Tile Numbering
When the tiles are imaged during the sequencing run, an output file is generated for each tile and named with
the tile number in a four digit format. With the exception of the nano flow cell, flow cells are imaged on the top
and bottom surface. The output files for each tile are located in the run folder in
Data\Intensities\BaseCalls\L001.
Flow Cell MiSeq Reagent Kit Tiles Imaging Surface
Image
File
Names
Standard Flow Cell
PGS Flow Cell
MiSeq Reagent Kits, v3 1-19 Top 1101
through
1119
1-19 Bottom 2101
through
2119
Standard Flow Cell MiSeq Reagent Kits, v2 1-14 Top 1101
through
1114
1-14 Bottom 2101
through
2114
Micro Flow Cell MiSeq Reagent Micro Kits, v2 1-4 Top 1101
through
1104
1-4 Bottom 2101
through
2104
Nano Flow Cell MiSeq Reagent Nano Kits, v2 1-2 Top only 1101
through
1102
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
52
MiSeq System Guide
Index
A
activity indicators 6
analysis
options 7
analysis workflow
definition 4
B
BaseSpace
connection 6-7
credentials 47
updates 14
BaseSpace Onsite
connection 7
credentials 47
server location 13
blinking icons 6
BlueFuse Multi software 7, 13
bundle logs 38-39, 41
C
cluster generation 18
CompletedJobInfo.xml 8
components
flow cell 9, 51
flow cell compartment 2-3
optics module 2
reagent cartridge 10
reagent compartment 2, 4
consumables 15
laboratory-grade water 16
control software 5
copying files and folders 38
customer support 57
cycles in a read 17
D
deleting files and folders 38
disk space
checking 8
low disk space 44
documentation 1, 57
domain name 47
E
email alerts 14
errors 6
F
flow cell
cap color 9
cleaning 21
letter designator 44
overview 9
single-lane 8
tile numbering 52
tiles 51
flow cell clamp 3
flow cell compartment 2-3
flow cell door sensor 6
fluidics
troubleshooting 45
washing 34, 36
folder locations
default settings 15
for current run 26
G
genome references 38
H
help, technical 57
I
icons
activity indicators 6
sensors 6
icons, blinking 6
idling the instrument 36
Illumina Proactive monitoring service 14
initialization 44
InterOp files 5
InterOp folder 50
IP address 47
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
53
L
laboratory-grade water guidelines 16
loading reagents
cartridge 24
PR2 23
Local Run Manager 1
M
maintenance wash 34
manifest file
copying to instrument 38
definition 4
MiSeq Reporter
overview 8
MiSeq Self-Service 44
monitoring the run 26
moving files and folders 38
N
network connection 44
network settings 47
O
optics module 2
P
password, changing 47
pausing a run 42
post-run wash 28
PR2, loading 23
R
read length 17
reagent cartridge 10
contents 10
inspect 19
thaw 19
reagent chiller, temperature 6
reagent compartment 2, 4
reagents
kitted 9
Real-time Analysis 1
results 51
run folder 49
template generation 27
Real-Time Analysis 5
recipes, managing 38
reference genome
file format 4
RFID
PR2 23
reagent cartridge 24
tracking 1
troubleshooting 44
RTAcomplete.txt 51
run duration 17
run folder
definition 4
run folders
contents 49
managing 38
naming 49
primary analysis files 51
temp, output, analysis 49
run options 13-15
run setup screens 21
RunInfo.xml 49, 51
runParameters.xml 49
S
sample sheet
changing 25
copying to instrument 38
definition 4
in run folder 51
not found 44
secondary analysis 8
sensor indicators 6
sequencing 18
Sequencing Analysis Viewer 8, 26
sequencing screen 26
shutting down the instrument 39
sipper handle 4
software
disk space checking 8
run duration 17
updating 39
software suite 5
software updating 14
standby wash 36
status.xml 51
stopping a run 43
support pages 1
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
54
MiSeq System Guide
system account name 47
system settings 47
T
technical assistance 57
template generation 8, 27
template line wash 28
tile numbering 52
training 1
troubleshooting
bundle logs 38-39, 41
fluidics 45
RFID 44
run-specific files for 41
run setup errors 44
U
Universal Copy Service 5
updating software 39
V
VeriSeq PGS workflow
flow cell 9
maintenance frequency 33
replicate analysis locally 13
secondary analysis 7
volume test 45
W
warnings 6
wash volumes 46
washes
benefit of 28
expected volumes 46
maintenance 34
post-run 28
post-run wash settings 13
prepare to idle 36
prepare to shut down 39
standby 36
waste bottle 4
workflow 18
run duration 17
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
55
MiSeq System Guide
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
56
MiSeq System Guide

Technical Assistance
For technical assistance, contact Illumina Technical Support.
Website:
www.illumina.com
Email:
techsupport@illumina.com
Illumina Customer Support Telephone Numbers
Region Toll Free Regional
North America
+1.800.809.4566
Australia
+1.800.775.688
Austria
+43 800006249 +43 19286540
Belgium
+32 80077160 +32 34002973
China
400.066.5835
Denmark
+45 80820183 +45 89871156
Finland
+358 800918363 +358 974790110
France
+33 805102193 +33 170770446
Germany
+49 8001014940 +49 8938035677
Hong Kong
800960230
Ireland
+353 1800936608 +353016950506
Italy
+39 800985513 +39 236003759
Japan
0800.111.5011
Netherlands
+31 8000222493 +31 207132960
New Zealand
0800.451.650
Norway
+47 800 16836 +47 21939693
Singapore
+1.800.579.2745
Spain
+34 911899417 +34 800300143
Sweden
+46 850619671 +46 200883979
Switzerland
+41 565800000 +41 800200442
Taiwan
00806651752
United Kingdom
+44 8000126019 +44 2073057197
Other countries
+44.1799.534000
Safety data sheets (SDSs)—Available on the Illumina website at support.illumina.com/sds.html.
Product documentation—Available for download in PDF from the Illumina website. Go to
support.illumina.com, select a product, then select Documentation & Literature.
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
57
Document # 1000000061014 v00
For Research Use Only. Not
for use in diagnostic procedures.
58
MiSeq System Guide

Illumina
5200 Illumina Way
San Diego, California 92122 U.S.A.
+1.800.809.ILMN (4566)
+1.858.202.4566 (outside North America)
techsupport@illumina.com
www.illumina.com
For Research Use Only. Not for use in diagnostic procedures.
© 2018 Illumina, Inc. All rights reserved.
Document #
1000000061014 v00
