
115 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Calotropis procera
: A phytochemical
and pharmacological review
Gaurav Parihar, Neelam Balekar*
IPS Academy, College of Pharmacy, Hukhmakedhi, Indore, Madhya Pradesh, India
ABSTRACT
Medicinal plants are used from the ancient time as the major sources of drugs. The fact is
that we can obtain many of the presently available drugs, either directly in the extract form
or in the modified synthetic form. Naturally, plants have the ability to synthesize products
beneficial for us namely as phytoconstituents that are used to perform biological functions,
which also protect us against predators such as virus fungi and other microorganisms. The
phytoconstituents obtained from the natural products are one of the most successful strategies
for the discovery of new drugs. Calotropis procera is a plant which is used in several traditional
medicine and folklore systems to cure various ailments as reported in the Hindu literature.
It is widely used in the Indian traditional medicinal system as well as in Arabic, Unani, and
Sudanese systems. C. procera is also used by various tribes of the world as a curative agent
for ailments such as skin disease and elephantiasis. Different parts of the plant have been
reported to possess various phytochemicals containing cardiotonic agents such as calotropin,
calotropagenin, calotoxin, calotropagenin and voruscharine, steroids, di and triterpenes such
as stigmasterol, β-sitosterol, flavonoids, polyphenolic compounds, and various newer reported
hydrocarbons and proteins. This shrub is known to possess a wide range of pharmacological
activities such as anticancer, acaricidal, schizonticidal, antimicrobial, anthelmintic, insecticidal,
anti-inflammatory, antidiarrheal, anticancerous, and larvicidal activities with other beneficial
properties. C. procera is small, erect shrub, which is used in several herbal and empirical
medicines to cure simple and deadly diseases and disorders. It is also reported widely in
various folklore preparations and ethnomedicines. This review is a profound attempt to stack
the information concerning pharmacognostical, phytochemical, and pharmacological features
of C. procera shrubs.
INTRODUCTION
C
alotropis procera (Arka) is an important drug in the
monograph of Ayurveda, and it is known in India
from the earliest time (Figure 1). It was mentioned by
Hindu writers and the ancient sacrificial rites many years ago.
There are two common species of Calotropis reported in the
literature, viz., C. procera (Ait.) R.Br. and Calotropis gigantea
(Linn.) R.Br. mentioned by the ancient writers. Both the species
consists of similar types of phytoconstituents discovered till
now and may be used as substitutes for one another might
have similar effects. Three varieties of Arka are mentioned
in the Hindu literature of Dhanvantari Nigantu as Suklarkah,
Rajarkah, and Sveta mandarah. It is widely used in the Indian
traditional medicinal system as well as in the other available
treatments such as Arabic, Unani, and Sudanese and for the
various diseases. C. procera is also used by various tribes of
the world as a curative agent for ailments such as skin disease,
elephantiasis, toothache, asthma, leprosy, and rheumatism [1].
Different parts such as leaves, roots and bark, flower, fruits, stem,
and latex of the plant have been reported to possess various
phytochemicals which might possess various pharmacological
activities. The coarse shrub possesses acaricidal, schizonticidal,
antimicrobial, anthelmintic, insecticidal, anti-inflammatory,
antidiarrheal, anticancerous, and larvicidal activities with
other beneficial properties [2]. The plant is described as a
golden gift for humankind containing cardiotonic agents such
as calotropin, calotropagenin, calotoxin, calactin, uscharin,
amyrin, amyrin esters, uscharidin, coroglaucigenin, frugoside,
corotoxigenin, calotropagenin, and voruscharine used in the
therapeutic treatment [2].
Different compounds such as norditerpenic esters, organic
carbonates, the cysteine protease procerain, alkaloids, flavonoids,
sterols, and numerous cardenolides made this plant of scientific
attraction for centuries. Hence, in this review, an account of
reported pharmacological actions of the plant with reported
active chemical constituents were discussed in this study.
Review Article
Corresponding Author:
Dr. Neelam Balekar,
IPS Academy, College of
Pharmacy, Hukhmakedhi,
Rajendra Nagar, A.B.
Road, Indore - 452 012,
Madhya Pradesh, India.
Phone: +91-9893405071,
Fax: +91-7314041627.
E-mail: neelambalekar@
gmail.com
Received: Apr 8, 2016
Accepted: Aug 29, 2016
Published: Sep 26, 2016
Keywords:
Arka, phytoconstituents,
plant pharmacology,
traditional medicine

Parihar and Balekar: C. Procera: A review
116 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
DESCRIPTION
Habitat
C. procera favors open habitat with little competition. The plant
of this species grows in dry habitat where rainfall is limited
to 150 to 1000 mm and also found in the area of excessive
drained soil as much as 2000 mm of annual precipitation. It
is also found in the common habitat of road-side, beachfront
dunes, and widely disturbed in the urban areas. C. procera is
also found at the elevated areas up to 1,000 m. Because the
plant is easy to propagate and manages and can grow under
the xerophytic condition, sometimes it is also grown as an
ornamental plant in dry or coastal areas [2,3].
Geographical Distribution
C. procera is inborn to Southern Asia and Indo-China to
Malaysia, Macaronesia, West Africa North and East Africa,
Madagascar, and Arabian Peninsula. The plant is naturalized
in Australia, Central America, North, South America, and
West Indies. The species is now accepted and culture in many
countries such as Mexico, Central and South America, Pacific
islands, Australia, and the Caribbean [2,4].
Scientific Classification
Taxonomy Calotropis procera (Ait.) Ait.f. Kingdom:
Plantae – Plants; Subkingdom: Tracheobionta – Vascular
plants; Superdivision: Spermatophyta – Seed plants;
Division: Magnoliophyta – Flowering plants; Class:
Magnoliopsida – Dicotyledons; Subclass: Asteridae; Order:
Gentianales; Family: Asclepiadaceae; Genus: Calotropis
R.Br. – Calotropis; Species: C. procera (Ait.) Ait.f. [1].
Synonyms/Other Latin Names
Asclepias procera Aiton, common vernacular names (Sanskrit)
Arka, (Hindi) Aaka. Giant Indian Milkweed. Sodom Apple,
Small Crown Flower, Rooster tree, French Cotton in English.
Remiga (Malaysia), Dok Hak (Laos), Kapal-kapal (Philippines),
Nam t[it] b[at] (Vietnam), Pomme de Sodome (French), Rubik
(Indonesia), Mudarpflanzer (German), Algodon Extranjero
(Spanish), Ipekag (Turkish), Oshar (Arabic), Calotropo
(Italian), Po Thuean, Paan Thuean (northern), and Rak
(central) in Thailand [1,2,5].
Botanical Description
The plant is an evergreen, soft-wooded, perennial shrub; small
tree attains a maximum height up to 2.5 m (maximum 6 m).
A copious amount of white sap generates whenever any part
of the plant is cut. The bark is corky, furrowed, and light gray.
The root is simple, branched, and woody at base and covered
with a fissured, corky bark, branches has very deep stout
root with few branches. The leaves are opposite-decussate,
simple, subsessile, and exstipulate; the leaves are slightly
leathery and having a fine coat of soft hairs that sometimes
sting too. Flowers are shallow bell-shaped, like a campanula,
bracteate, complete, bisexual, actinomorphic, pentamerous,
hypogynous, pedicellate, multiflowered, umbellate, peduncled
cymes with axillary or terminal inflorescence. Five sepals, 5
lobed shortly united that are 4-5 mm long. Five-lobed petals
(Corolla), gamopetalous, twisted aestivation. Androecium
has five stamens, gynandrous, anther dithecous, coherent.
Gynoecium is bicarpellary, apocarpus, and styles are united at
their apex, peltate stigma with five lateral stigmatic surfaces.
Anthers are adnate to the stigma forming a gynostegium. Fruit
is simple, fleshy, inflated, and subglobose to obliquely ovoid
follicle. Seeds are present in large amount, small, flat, obovate,
compressed with silky white pappusat the one end, 3 cm or
more long [1,2,5,6].
Ethnomedical (Traditional) Uses
The leaves were reported to use in sun worship from the Vedic
times. Secretions from the root bark were used by Hindu
physicians to treat skin diseases, cough, intestinal worms,
ascites, and anasarca and also in enlargements of abdominal
viscera, etc. The milky juice was considered as a drastic
purgative and caustic. Flowers were considered to improve
digestion, catarrh, and increase appetite. The root bark was
also used to treat elephantiasis. Calotropis latex is used and
applied intact in the preparations for toothache. The flowering
tops were also used to treat asthma. The plant was also used
in the treatment of leprosy, hepatic, and splenic enlargements.
The leaves were boiled, and oily preparations were made
and used in the treatment of paralysis. Leaf powder was
considered as a substitute for ipecacuanha and also possesses
the properties of Gutta-persica also used in wound healing.
The juice was used for the purpose of infanticide and was
sometimes given to women to induce abortion. Tanners used
the milky juice to remove hair from hides [2,7].
Pear-shaped fruit and latex have medicinal properties.
The raw latex is often considered poisonous, but reports of
its toxicity may be exaggerated. A safe, effective dose could
be obtained by scooping out the seeds and pulp from a halved
ripe fruit and drinking sheep, goat, or camel milk from the
remaining green skin “cup.” Poultices made from the leaves
used to heal rheumatism. Levey identifies the Sodom apple
with Ladanum asclepiad, which Al-Kindi used in a dentifrice,
for lengthening the hair, and in a formula for exterminating
worms and purifying the air during an epidemic [8].
The powder of the root mixed with milk of goat is used
in epilepsy; route of application is in the nostrils. The tribes
of the Varanasi use latex to remove worms from teeth and in
the preparations of toothache. Traditionally, C. procera bark is
used to treat cholera, extracting Guinea worms, and digestion.
The drug is well known to enhance bile secretion and has a
sedative effect on intestinal muscles. The tender leaves are
also used to cure migraine. An ethnomedicinal profile of
different plant parts of C. procera was compiled by Verma
et al., 2010 [9].
PHYTOCHEMICAL REPORTS
A vast number of research and review articles are published
on the phytochemical and screening properties of C. procera.
All parts of the plant have toxic potential, due to the presence
of cardenolides (cardiac glycosides). The latex was found
to be richest in cardenolides, which is already mentioned in
the literature. According to research, the leaf of the plant
consists of cardenolides 162 mg/g at dry weight and 2 mg/g

Parihar and Balekar: C. Procera: A review
117 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
total dry weight. The important cardenolides found in the
plant are voruscharin, uscharidin, uzarigenin, calotroposide,
calactin, calotoxin, uscharin, ascleposide, calotropagenin,
coroglaucigenin, calotropin, proceroside, proceragenin,
and syriogenin. Many of these compounds formed in the
mechanism of extraction when hydrolyzed in a chemical
reaction. Latex differs in the quantities of cardenolides from
the other plant parts stem, fruit, leaves, and root bark. The
main cardenolides in the various parts of the plant are uscharin
and calotropagenin in the latex; calotropin and calotropagenin
in the leaves; uscharidin, calotropin, proceroside, and
calactin in the stem; calotoxin and calactin in the root bark;
coroglaucigenin and uzarigenin in the fruit pericarp. The seeds
contain 0.23-0.47% cardenolides, mainly coroglaucigenin or
frugoside [10].
Besides the cardenolides, other phytochemicals are also
reported from the plant such as sterols, flavonoids, coumarins,
alkaloids, triterpenes, saponins, tannins, and hydrocarbons
were isolated from the plant. The major flavonoid is rutin
(quercetin-3-rutinoside): Roots contain 1.7%, stem 4.8%,
leaves 5.0%, flowers 7.6%, and latex 9.7%. The plant is also
reported to contain resins, fatty acids, proteases, hydrocarbons,
amino acids, and many minerals. The polyphenol content
in different plant parts varies from 3.3% (leaf) to 4.9%
(stem) [11].
The flowers mainly contain α-and β-amyrins, an alkaline
phosphate, cyaindin-3-rhamnoglucoside, cycloart-23-en-3β,
25-diol, cyclosadol, multiflorenol, procestrol, quercetin-3-
rutinoside, β-sitosterol, β-sitost-4en-3one, and stigmasterol.
Cyanidin-3-rhamnoglucose and the triterpene calotropenyl
acetate are found in the flowers [12].
The leaves contain ascorbic acid, calactin, calotoxin,
calatropagenin, calotropin, polysaccharide containing
D-arabinose, D-glucose, D-glucosamine and L-rhamnose,
calotropagenin, and 3-proteinase. The latex contains
calotropin, α-calotropeol, 3-epimoretenol, gigantin, giganteol,
isogiganteol, α-lactuceryl acetate, α-lactuceryl isovalerate,
lupeol, proceroside, proceragenin, syriogenin, taraxast-20α-
(30)-en-(4-methyl-3-pentenoate), 3’-thiazoline cardenolide
uscharidin, uzarigenin, voruscharin and β-sitosterol, powerful
bacteriolytic enzyme in latex [13]. The latex contains 11-23%
rubber, the triterpenoids α- and β-amyrin, lupeol, taraxasteryl
acetate, α-and β-calotropeol, 3-epimoretenol, multiflorenol,
cyclosadol, several triterpene esters, the sterols β-sitosterol
and stigmasterol, the non-toxic cysteine proteases calotropin,
procerain and procerain-B and the alkaloid choline [13].
The root-bark contains benzolisoleneolone,
benzollineolone, long-chain fatty acids, and C (18) isoursane.
The plant also reported to contain calactinic acid, choline and
O-pyrocatechuic acid, β-sitosterol, taraxasterol, its φ-isomer:
taraxasteryl isovalerate and taraxasteryl acetate [14]. The
Presence of four new ursane-type triterpenes: Vrsa-13(18),
19(29)-diene-3α-yl-acetate, 18αH-urs-19(29)-en-3-one,
18αH-ursa-12, 20(30)-diene-3 α -yi-acetate and 18αH-urs-12-
en-3α-ol, were reported from the root bark [15]. Mudarine
as principal cardioactive constituent present in the leaves
is reported by Chaudhari [16]. Carruthers isolated and
characterized isorahamnetin-3-O-rutinoside, isorahamnetin-
3-O-glucopyranoside and taraxasteryl acetate, flavonoids from
Calotropis [12]. A yellow resinous substance from root bark
was also found by Sharma [17]. From the root bark, several
digitanol glycosides were isolated, which lack cardiac activity.
Four new ursane-type triterpenes calotroprocerol
A, calotroproceryl acetate A, calotroprocerone A, and
calotroproceryl acetate B from the root bark of C. procera
were isolated and structure elucidated in addition to five
known compounds [18]. Two labdane-type di terpenic
galactosides have been isolated for the first time from the
roots of C. procera, and structures are established as Labdan-
18-ol-β-D-galactofuranoside and Labdan-3 β-ol-11, 15-olide-
18,20-dioic acid-3 β-D-galactofuranoside [19]. In a study,
phytoconstituent of leaves hexane extract of C. procera was
investigated qualitatively and quantitatively by GC-MS. 12
major phytocompounds were identified and estimated. The
highest peak area was obtained by Ergost-5-en-3-ol (C
28
H
48
O),
and the lowest peak area was obtained by 9 octadecenoic acid
9-Octadecenoic acid (Z)-(C
18
H
34
O
2
) [20].
The ethyl acetate fraction of the methanolic extract
of the root barks of C. procera (Asclepiadaceae) resulted in
the identification of a new cardenolide glycoside named
proceraside A [21]. Three new cardenolides, along with eight
known ones, were isolated from the latex of C. procera [22].
Two new cardenolides, named ischarin and ischaridin, were
isolated from C. procera Ait. (Asclepiadaceae) [23]. The
n-BuOH fraction of the root bark of C. procera (Ait) R.Br. Seven
new oxypregnane oligoglycosides: Calotroposides H-N (1-7)
were isolated and identified [24].
Beside this, various parts of the plant possess
various phytochemicals reported till date. Various newer
phytochemicals reported till now; Table 1 shows the chemical
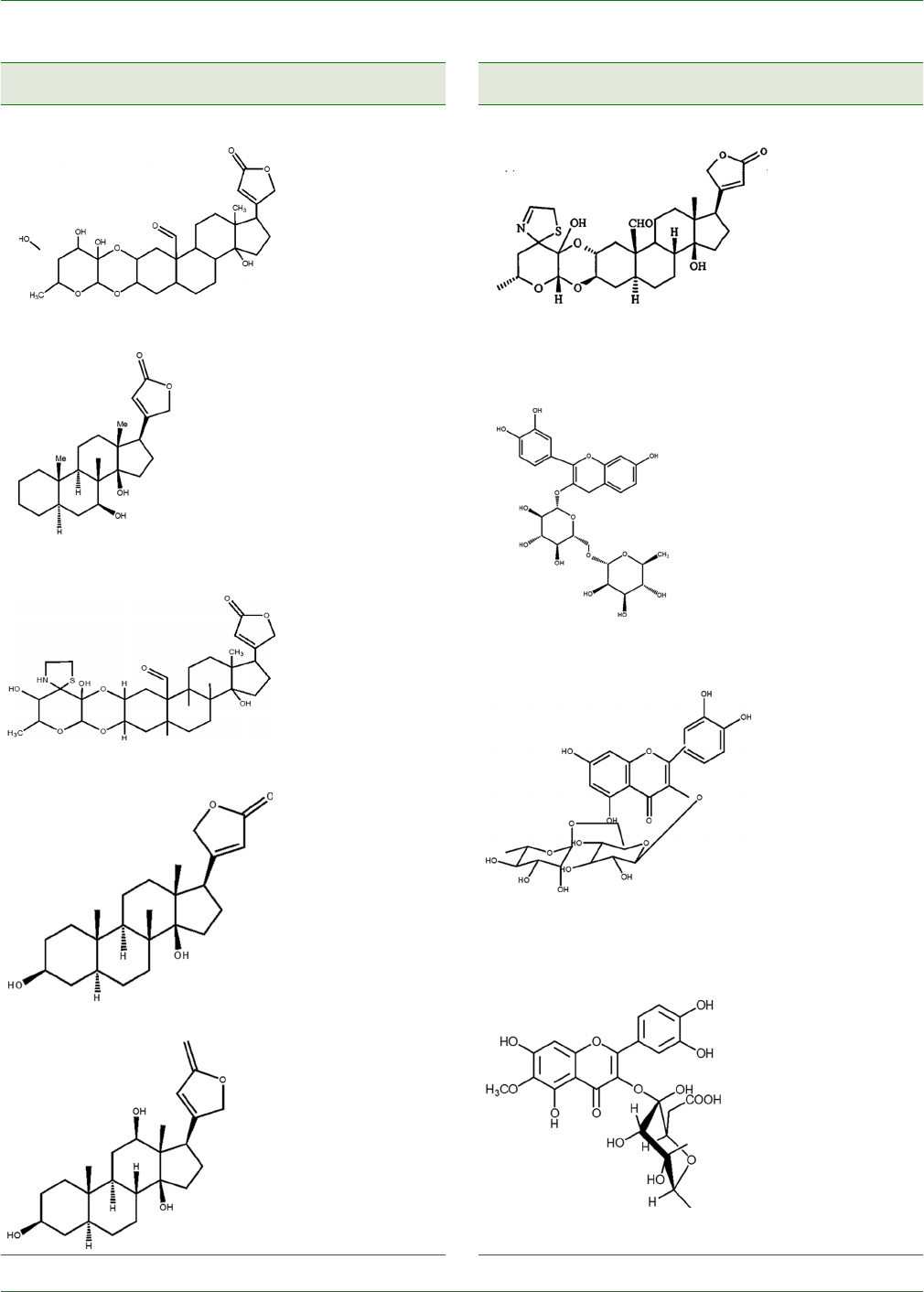
structures of phytoconstituents present in C. procera.
PHARMACOLOGICAL ACTIVITIES
The literature of the plant revealed us that various parts of
the plant such as root bark, stem bark, leaf, flower, and latex
and their extracts, fraction, and isolated compound showed
significant anticoagulant, antidiarrheal, anti-inflammatory,
antioxidant, antiulcer, analgesic, cough-suppressing,
hepatoprotective, smooth muscle-contracting, neuromuscular
blocking, spermicidal, and wound healing activity. Various
pharmacological activities of the plant parts reported on
Calotropis procera are shown in Table 2.
Analgesic and Antinociceptive Activity
In this study, analgesic activity of dry latex (DL) of C. procera
has evaluated. The effect of DL at a dose of 415 mg/kg
against acetic acid-induced writhing was more pronounced
as compared to an oral dose of aspirin (100 mg/kg). DL
(830 mg/kg) produced marginal analgesia in tail-flick model
which was comparable to aspirin [25,26].
Antinociceptive effect of proteins from the C. procera latex
using three different experimental models of nociception - acetic
acid, formalin-induced abdominal constrictions, and hot plate
test in mice - was evaluated. The latex protein fraction at the
doses of 12.5, 25, and 50 mg/kg showed the antinociceptive
effect in a dose-dependent manner, which is independent of
the opioid system [27,28].

Parihar and Balekar: C. Procera: A review
118 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
(Contd...)
Compound names Plant
part
References
Steroid
Procesterol
(24S)-24-ethyl-stigmast-
4-en-6a-ol-3-one
Flower [10]
Ergost-5-en-3-ol Leaves [41]
ß-Amyrin Root
bark
[26]
α-Amyrin Root
bark
[26]
Calotropin
12β-O-benzoyl-3β,14β,17β-
trihydroxypregnane 20-one
Roots [42]
Table 1: Secondary metabolites of Calotropis procera (R.Br.)
Compound names Plant
part
References
Taraxasterol Root
bark
Leaves
[42]
α-amyrin acetate
Urs-12 -en-3 -olyl acetate
Roots [26]
ß-Amyrin acetate Root
bark
[26]
Proceraursenolide
18 a H - urs - 1 2 en - 3 , 25 - olide
Roots [43]
Calotroprocerone-A
ursa-5,12,20(30)-trien-18aH-3-one
Root
bark
[44]
Table 1: (Continued)
(Contd...)

Parihar and Balekar: C. Procera: A review
119 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
(Contd...)(Contd...)
Compound names Plant
part
References
DI and triterpenes
Calotropenyl acetate
Urs-19(29)-en-3-ol, acetate, (3beta)
Root
bark
[44]
Gofruside
Corotoxigenin 3-O-β-D-
allomethyloside)
Roots [42]
Lupeol Root
bark
[43]
3b,27-dihydroxy-urs-18-en-
13,28-olide
Latex [45]
urs-19(29)-en-3-yl acetate Latex [45]
Table 1: (Continued)
Compound names Plant
part
References
β-Sitosterol Latex [45]
Stigmasterol Latex [45]
Stigmasta-5,22-dien-3-ol Leaves [20]
Multiflorenol
urs-19(29)-en-3-b-ol
Latex [45]
3b,27-dihydroxy-urs-18
-en-13,28-olide
Latex [45]
Table 1: (Continued)

Parihar and Balekar: C. Procera: A review
120 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
(Contd...)
Table 1: (Continued)
Compound names Plant
part
References
Procerursenyl acetate
urs-18a-H-12, 20 (30)-diene-3ß-yl
acetate
Roots [26]
Benzoyllineolone Root bark [26]
Diterpene
3,7,11,15 tetramethyl hexadecanyl
6′-methyl hept-5′-enyl ether (phytyl
isooctyl ether)
Roots [46]
Benzoylisolineolone Root bark [26]
Diterpene
3,7,11,15 tetramethylhexadecanoyl
-β-D-glucopyranosyl
-(2→1)-β-D-glucopyranosyl-(2→1)-β-
D-glucopyranosyl(2→1)-β-D-
glucofuranoside
(dihydrophytoyl tetraglycoside)
Roots [46]
Compound names Plant
part
References
Procerasesterterpenoyl
triglucosideDiterpene
2,6,10,14,18-
pentamethylnonadecanoyl‑β-D
glucopyranosyl-(2→1)-
β-D-glucopyranosyl
-(2→1)-β-D-glucopyranoside
Roots [46]
Uscharidin Latex [26]
18 H-urs-12, 2 0(30)-dien-3-yl acetate Roots [43]
Calotroprocerol–A
ursa-5,12,20(30)-trien-18aH-3b-ol
Root
bark
[44]
Urosolic acid Leaves [25]
Table 1: (Continued)
(Contd...)

Parihar and Balekar: C. Procera: A review
121 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Compound names Plant
part
References
Calotroproceryl acetate A
ursa-5,12,20(30)-trien-18aH-3b-yl
acetate
Root
bark
[44]
Pseudo-taraxasterol acetate Root
bark
[44]
Calotropursenyl acetate-B Root
bark
[44]
Terpenoid glycosides
bisabolan-11,14-diol-14-b-D
-glucopyranosyl-(1→2)
-b-Dglucopyranoside
Roots [43]
2-limonenyloxybenzoyl-
1β-D-glucopyranosyl
-(1→2)-β-D-glucopyranosyl-(1→2)
-β-D-glucuronopyranosyl-(1→2)-β-D
-glucuronopyranoside
Roots [43]
Table 1: (Continued)
Compound names Plant
part
References
1,2-dihexadecanoyl -3-phosphatyl
glycerol
Roots [43]
Polyphenolic compounds
Gallic acid Whole
plant
[47]
(−) Epicatechin Whole
plant
[47]
Ferulic acid Whole
plant
[47]
p-coumaric acid Whole
plant
[47]
Vanillic acid Whole
plant
[47]
n-tetradecanyl n-hexadec-9-enoate
(n -tetradecanyl palmitoleate)
Roots [43]
Table 1: (Continued)
(Contd...) (Contd...)

Parihar and Balekar: C. Procera: A review
122 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
(Contd...)
Compound names Plant
part
References
Hydrocarbons
(E)-Octadec-7-enoic acid Root
bark
[44]
4-hydroxy-4-methylpentan-2-one Latex [48]
2,3,4-trimethylhexane Latex [48]
Decane Latex [48]
n-Pentadecane Latex [48]
2,6 dimethyl tetra-1,5-decaene Latex [48]
n-Eicosane Latex [48]
3,7,11-Trimethyl-2,6,10,12
-pentadecatrien-1-ol
Latex [48]
2,6,10,15,19,23-Hexamethyl
-2,6,10,14,18,22-tetracosahexaene
Latex [48]
1,3,5-Triisopropylbenzene Latex [48]
Napthalene decahydro2,6 dimethyl Leaves [20]
2-H Benzofuranone 5,6,7, 7A
tetrahydro 4,4,7A trimethyl
Leaves [20]
6,10,14-trimethyl, Pentadecanone -2 Leaves [20]
Table 1: (Continued)
Compound names Plant
part
References
Hexadaconic acid, methyl esters Leaves [20]
9-Octadecenoic Acid (Z)- Leaves [20]
9,12,15-Octadecatrienoic acid, methyl
ester, (Z, Z, Z)-2-Hexadecen-1-ol,
3,7,11,15-tetramethyl-,
[R-[R*, R*-(E)]]-
Leaves [20]
(6Z), (9 Z) Pentadecadien 1-ol Leaves [20]
Farnesol isomer Leaves [20]
Tetratetracontane Leaves [20]
Proceranol
n-triacontan -10ß-ol
Roots [29]
N-dotriacont-6-ene Roots [29]
Glyceryl mono-oleolyl-2-phosphate Roots [29]
Table 1: (Continued)
(Contd...)

Parihar and Balekar: C. Procera: A review
123 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
(Contd...)
Compound names Plant
part
References
Methyl myrisate, Roots [29]
Methyl behenate Roots [29]
(E)-3-(4-methoxyphenyl-2-O-beta
-D-4C1-glucopyranoside)-methyl
propenoate
Leaves [49]
Ž2‑propenyl‑2‑hydroxyethyl
carbonate
Latex [50]
Acetic acid Root
bark
[26]
Isovaleric acid Root
bark
[26]
Choline Latex [26]
Cardenolides
Cardenolide
2’’-oxovoruscharin
Root
barks
[41]
Table 1: (Continued)
(Contd...)
Table 1: (Continued)
Compound names Plant
part
References
Calotropagenin Leaves [25]
Calotropin Latex [25]
Calotoxin Latex [25]
Calactin Latex [25]
Voruscharin Latex [25]
Parihar and Balekar: C. Procera: A review
124 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Compound names Plant
part
References
Proceroside Flower [25]
Proceragenin Flower [25]
Voruscharin a-calotropeol Flower [25]
Uzarigenin Latex [26]
Syriogenin Latex [26]
(Contd...)
Table 1: (Continued) Table 1: (Continued)
Compound names Plant
part
References
Uscharin Latex [26]
Flavonoids
Quercetin-3 rutinoside Latex [26]
Quercetin 3-O-rutinoside Stem,
leaves
and
flower
[51]
Quercetagetin-6-methyl ether
3-O-beta-D-4C1-galacturonopyranoside
Leaves [49]
(Contd...)

Parihar and Balekar: C. Procera: A review
125 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Compound names Plant
part
References
Isorhamnetin-3-O-rutinoside Leaves [26]
Isorhamnetin-3-O-robinobioside Leaves [26]
Carbohydrates
D-arabinose Flower [26]
Glucose Flower [25]
Glucosamine Flower [25]
L-rhamnose Flower [25]
α-rhamnose Leaves [26]
Table 1: (Continued)
Anticonvulsant Effects
The anticonvulsant activity by maximal electroshock seizures
(MES), pentylenetetrazol (PTZ), lithium-pilocarpine, and
electrical kindling seizures of C. procera root aqueous and
chloroform extracts in rats was performed [29]. In the MES
test and the PTZ test, the chloroform extract showed a highly
significant effect. Both the extracts also inhibited convulsions
induced by lithium-pilocarpine and electrical kindling [26].
Antimalarial Activity
The ethanolic extracts of the different parts of C. procera
showed IC
50
values ranging from 0.11 to 0.47 mg/ml against
Plasmodium falciparum MRC20 CQ-sensitive strain and from
0.52 to 1.22 mg/ml against MRC 76 CQ-resistant strain,
flower, and bud extracts being the most effective. Although
220, 440 times less effective than CQ, these extracts deserve
further studies aimed at the identification of the active
constituents [25].
Anthelmintic Activity
The anthelmintic activity of C. procera flowers in comparison
with levamisole was evaluated through in vitro and in vivo
studies on live Haemonchus contortus. In the in vitro study
crude aqueous (CAE) and crude methanolic (CME) extracts,
and for in vivo study, CAE, CME extracts and crude powder
(CP), of flowers were used. Egg count percent reduction was
recorded as 88.4% and 77.8% in sheep treated with CAE and
CP at 3 g/kg
-1
; CME was found least effective in (20.9%)
reduction in ECR. All the extracts exhibited lower activity than
that exhibited by levamisole (97.8-100%). Cavalcante et al.
evaluated the chemical composition and in vitro activity of
latex on H. contortus [26,28,30].
Antioxidant and Antidiabetic Activity
The antioxidant activity of dried latex (DL) of C. procera and
antidiabetic effect against alloxan-induced diabetes rats was
evaluated. The oral dose of DL at 100 and 400 mg/kg was
administered. The result revealed us that there is decrease in
blood glucose and increase in the hepatic glycogen content. Tsala
et al. evaluated the antioxidant activity of the ethanol extract of
C. procera bark against surgical wounds [25,28,31,32].
Myocardial Infarction
C. procera latex was evaluated for protection against
isoproterenol (20 mg/100 g)-induced myocardial infarction
in albino rats. The pretreatment of ethanolic latex extract at
a dose of 300 mg/kg orally three times a day for 30 days,
significantly reduces elevated marker enzymes (serum
glutamic-pyruvic transaminase, serum glutamic oxaloacetic
transaminase, and alkaline phosphatase) level in serum and
heart homogenates [28].
Schizontocidal Activity
The effect of crude fractions of flower, bud, and root against
a chloroquine sensitive strain, MRC 20 and a chloroquine
resistant strain, MRC 76 of P. falciparum were evaluated.
The effectiveness of its fractions was compared with the
CQ-sensitive strain than the CQ-resistant strain in vitro [28].

Parihar and Balekar: C. Procera: A review
126 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Anticancer and Cytotoxic Properties
The anticancer and cytotoxic properties of the DL of C. procera
in transgenic mouse model of hepatocellular carcinoma
were performed and found complete protection against
hepatocarcinogenesis. There was a significant lowering of
serum vascular endothelial growth factor level and extensive
cell death in both Huh-7 and COS-1 cells while AML12 cells
were found live. This was accompanied by extensive
fragmentation of DNA in Huh-7 and COS-1 cells. No change
in the levels of Bcl
2
and caspase 3 was observed; these are
the canonical markers of apoptosis. Gurung et al. found of
the anticancer bioactive compound proceraside by molecular
docking with macromolecules involved in the cell cycle and
DNA replication [28,31,33,34].
Antimicrobial Activity
The antimicrobial activity of the leaf extracts of C. procera
was evaluated, and the inhibitory effect of extract of latex of
C. procera against Candida albicans was observed [25,26]. The
antibacterial activity of a new cardenolide, 7B, 14B-dihydroxy-
5-card-20(22) enolide (proceragenin) of C. procera was
evaluated [26] which was found to be active against
Pseudomonas pseudomallei, a causative agent of melioidosis.
All the leaf extract fractions completely inhibited the growth of
the tested organisms. The antimicrobial activity of C. procera
was evaluated against some of the tested microorganisms
(Staphylococcus aureus and Pseudomonas aeruginosa, and one
pathogenic fungus, C. albicans) [27].
The antimicrobial effect of ethanol, aqueous, and
chloroform extracts of leaf and latex of C. procera were
studied on five bacteria, namely, Escherichia coli, S.
aureus, Staphylococcus albus, Streptococcus pyogenes, and
Streptococcus pneumoniae and three fungi: Aspergillus niger,
Aspergillus flavus, and Microsporum boulardii and one yeast
C. albicans using agar well diffusion and paper disk methods
[28]. The results revealed that ethanol was the best extractive
solvent for antimicrobial properties of leaf and latex of C.
procera followed in order by chloroform and aqueous. The
ethanolic extracts of C. procera latex gave the widest zone of
inhibition (14.1 mm) against E. coli using agar well diffusion
while 9.0 mm was recorded for the same organism in the
disc plate method. The growth of six bacterial isolates was
inhibited by the three extracts except P. aeruginosa and S.
pyogenes that were not inhibited by the aqueous extracts of
both leaf and latex of C. procera. Similarly, the growth of four
test fungi was inhibited by ethanol and chloroform extracts
while the aqueous extract was the least effective on the test
fungi [26].
Anti-inflammatory Activity
The latex (DL) of the plant C. procera has been reported to exhibit
potent anti-inflammatory activity against carrageenan and
formalin that are known to release inflammatory mediators. The
anti-inflammatory effect of aqueous and methanolic extracts of
DL was more pronounced than phenylbutazone (PBZ) against
carrageenan, whereas it was comparable to chlorpheniramine
and PBZ against histamine and prostaglandin E
2
, respectively.
Both extracts produced about 80%, 40%, and 30% inhibition
of inflammation induced by bradykinin, compound 48/80, and
serotonin. The histological analysis revealed that the extracts
were more potent than PBZ in inhibiting cellular infiltration
and subcutaneous edema [35]. A single dose of the aqueous
suspension of the DL was effective to a significant level against
the acute inflammatory response. The crude DL of C. procera
possesses a potent anti-inflammatory activity [33].
The effect of methanolic dried extract MeDL was compared with
PBZ a non-selective cyclooxygenase (COX) inhibitor, rofecoxib,
a selective COX-2 inhibitor. MeDL of C. procera markedly
reduces cell influx, release of mediators, and oxidative stress
associated with arthritic condition, and therefore, has the
potential to be used as an antiarthritic agent. Chaudhary et al.
reported a protective effect of high molecular weight protein
sub-fraction of latex in monoarthritis rats [28,36].
Larvicidal Activity
C. procera was tested against Anopheles labranchiae mosquito
larvae and exhibited high larvicidal activity with LC
50
(24 h)
ranging from 28 to 325 ppm [26]. The giant milkweed was
effective in both inhibition of feeding and causing mortality
of larvae. The different rubber-free fractions of the latex were
evaluated against egg hatching, and larval development of the
mosquito Aedes aegypti was found inhibitory effect [27].
The effects of alkaloid extracts of C. procera leaves at the
vegetative stage on the survival of fifth instar larvae and on the
ovarian growth of Schistocerca gregaria have been studied [28].
The toxic effects of crude extracts (both for leaves and flowers)
of C. procera against two species of termites, i.e. Heterotermes
indicola and Coptoter mesheimi were studied [26]. Similarly, C.
procera showed moderate larvicidal effects against second and
fourth instar larvae of the laboratory-reared mosquito species,
Culex quinquefasciatus [25]. C. procera appears to be more
effective than Haloxylon recurvum and Azadiracta indica [26].
Immunomodulatory Activity
Ethanolic extract of the root bark of C. procera was evaluated
for immunomodulatory activity using immunological tests
in mice, humoral mediated antibody titer, delayed-type
hypersensitivity, peritoneal macrophage count, vascular
permeability, hematological profile, i.e. total red blood cell
count, total leukocyte count, % neutrophils and % lymphocytes,
and cyclophosphamide-induced myelosuppression
at three dose levels (50, 100, and 200 mg/kg). The
extract stimulates defense system by modulating several
immunological parameters. Nascimento et al., 2016 discover
immunomodulatory properties of latex protein extracts from
C. procera which protect against experimental infections with
Listeria monocytogenes [37-39].
Wound Healing Activity
Based on its traditional use, C. procera was selected for
evaluation of its wound healing potential in Guinea pigs. 20 μl
of 1.0% sterile solution of the latex of the plant in the animals
was applied topically. The latex significantly augmented the
healing process by markedly increasing collagen, DNA and
protein synthesis and epithelization. Tsala et al. evaluated

Parihar and Balekar: C. Procera: A review
127 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Part of plant Activity References
Root, latex flowers Analgesic, antinociceptive, antipyretic activity [25,27]
Latex
Plant
Anthelmintic activity
Haemonchus contortus, Schistosoma mansoni,
Rhipicephalus (Boophilus) microplus, Ascaris
[28,26,30]
Latex Antiarthritis and monoarticular arthritis model [2,36]
Root Antiangiogenesis [26]
Flower
Leaf, latex
Antibacterial and antiparasitic antimicrobial activities [2,25,27,28,26]
Latex, root barks Anticancer and in vitro cytotoxicity hepatocellular carcinoma,
skin melanoma, Antitumor studies
[20,21,33-35,41]
Latex Anticonvulsant action [25]
Flowers Anticoccidial activity Eimeria tenella [26]
Latex Antidiabetic, diabetic wound, diabetic nephropathy, diabetic
neuropathy
[28,52,53]
Latex, ariel part Antidiarrheal activity [26]
Whole plant Antieczema, dermatophytic activity [2,40]
Latex Antiedematogenic [2,26]
Root, Flower, Latex Antifertility screening [26]
Leaf Antifilarial activity (Setaria digitata) [2]
Whole plant Antifungal activity (Ceratocystis paradoxa, Candida albicans) [26]
Leaves Antihyperbilirubinemic [2,53]
Leaves Anti-implantation activity [26]
Whole plant Antilithic [54]
Whole plant Antimycoplasmal activity [55]
Latex, root Antioxidant and free-radical scavenging activity [2,25-27,31,32]
Leaf Antiplasmodial activity [2]
Latex Antiseptic - Salmonella enterica s Typ [56]
Latex Antitermites property [26]
Leaves stem Antitussive activity [57]
Root, root bark, leaf, stem, latex Antitumor studies Antiproliferative and cell death (Apoptosis) [25,31]
Latex Allergic contact dermatitis Immunological and allergenic
responses, Immunomodulatory activity
[37-39]
Latex Asthma [35]
Latex Bullous eruption [35]
Latex Cardiotonic action [2,41]
Latex Clot inducing and dissolving properties [58]
Latex Cognition enhancer [59]
Aerial parts Effect on diverse muscles [60]
Latex Enzyme purification potential [25,41]
Latex Enzymatic activity [61]
Latex 5-fluorouracil-induced oral mucositis [62]
Latex, stem bark Gastric ulcers, gastric mucosal protective activity
Anti-Helicobacter pylori and urease inhibition
[2,26-28]
Leaf, flowers Glucose tolerance, hypoglycemic Effect [26]
Latex, flowers Hepatoprotective activity [26]
Latex Hepatorenal functions [63]
Latex Hemorrhagic septicemia or poisoning [26]
Table 2: Various pharmacological activities reported on the plant Calotropis procera
(Contd...)

Parihar and Balekar: C. Procera: A review
128 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
the antioxidant activity and the healing action of the ethanol
extract of C. procera bark against surgical wounds [27,32].
Antiulcer Activity
The antiulcer activity of C. procera using different in vivo ulcer
models was performed. The results of the study revealed that
it significantly inhibited aspirin, reserpine, absolute alcohol,
and serotonin-induced gastric ulcerations in rats and also
protecting the gastric mucosa from aspirin-induced ulceration
in pyloric-ligated rats, and significant protection was observed
in histamine-induced duodenal ulcers in Guinea pigs [26].
Antifertility Activity
The effect of an ethanolic extract of the roots of C. procera was
studied in albino rats to explore its antifertility and hormonal
activities. Strong anti-implantation (inhibition 100%) and
heterotrophic activity was observed at a dose of 250 mg/kg
(1∕4 of LD
50
). No antiestrogenic activity was detected [26].
Antidiarrheal Activity
The DL of C. procera was evaluated for its antidiarrheal activity.
Like atropine and PBZ, a single oral dose of DL (500 mg/kg) was
produced a significant decrease in the frequency of defecation
and the severity of diarrhea as well as protecting from diarrhea
in 80 % rats treated with castor oil [26].
Estrogenic Functionality
The effects of ethanolic and aqueous extracts of C. procera roots
were studied on the estrous cycle and on some parameters of
estrogenic functionality in rats. Both extracts were found to
interrupt the normal estrous cycle in 60% and 80% of rats
treated [2,26].
Part of plant Activity References
Latex Histaminic activity [2]
Latex Hyperalgesia effect [64]
Leaves Hypotensive [26]
Whole plant Insecticidal activity [2,26]
Latex Interleukin-1beta inducer [2]
Whole plant In‑vitro spasmolytic effect [27]
Leaves Lipolytic, lipoxygenase inhibitors [65,66]
Leaf
Latex
Larvicidal malaria, dengue/dengue hemorrhagic fever and
lymphatic filariasis-Musca domestica, mosquito larvae, Culex
quinquefasciatus say, Aedes aegypti, Anopheles stephensi
[25,26]
Latex Morphogenetic abnormalities [67]
Whole plant Molluscicidal activity [26]
Latex Myocardial infarction [28]
Root Estrogenic functionality [26]
Latex Ontogenetical and histochemical [2]
Aerial parts Purgative [2]
Root, latex, flowers Pro- and anti-inflammatory activities acute inflammation [2,26-28]
Latex Pleurisy [2,25,27]
Latex Proteolytic enzyme activity [61]
Latex Prostaglandins releaser [2]
Arieal part Reproductive potential [68]
Whole plant Schizontocidal activity [2,28]
Latex Toxicity study - Toxic iridocyclitis keratoconjunctivitis. corneal
endothelial cytotoxicity ocular toxicity keratitis cytostatic and
cytotoxic activity, dermatophytes
[25-26,35]
Latex, leaves, bark Wound healing, antikeloidal activity, and surgical wounds [27,32]
Table 2: (Continued)
Figure 1: Photograph of plant Calotropis procera; flowering shoot,
inflorescence, stem, leaves

Parihar and Balekar: C. Procera: A review
129 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
Dermatophytic Activity
Fresh latex of C. procera was screened for antifungal activity
against dermatophytes: Trichophyton spp., Microsporum spp.,
and Epidermophyton spp. The result shows Trichophyton spp.
being the most susceptible followed by the Microsporum spp.
and Epidermophyton spp. were least inhibited [40].
Toxicity Studies
The plant is proven as toxic, and it is one of the plants not
eaten by grazing animals. The latex from the plant has used
by the tribal people to make poison arrows used for hunting
purpose. The latex is highly toxic to human eyes cause ocular
toxicity and produces loss of vision with photophobia. Latex of
C. procera was studied for its inflammatory effects using pedal
edema and air pouch models of inflammation in rats and could
be used to evaluate anti-inflammatory drugs. Furthermore,
latex also produces toxic iridocyclitis, keratoconjunctivitis,
corneal endothelial cytotoxicity, and keratitis when applied
accidentally on the eye.
In a study, DL and flowers of C. procera and its ethanolic
extracts were evaluated against MCF-7 and HeLa cell line
cultures against the MTT assay to determine the inhibitory
effects of test compounds on cell growth in vitro. The standard
drug tamoxifen inhibits 60.46% breast cancer (MCF-7) cells,
whereas the ethanolic extract of DL and flowers showed
cytotoxic properties against both MCF-7 and HeLa cells in a
dose-dependent manner [2,26-28].
CONCLUSION
The plant Calotropis is one of the widely distributed along the
world geographical area. The whole summation of information
about the use of C. procera in the entire world is matched with
available literature. It is well mentioned in the Indian materia
medica; there is broad categorization according to its various
uses in the pharmacological as well as in traditional use. The
literature showed us that it is the plant that is forgotten as
the time passes. Still many scientists have worked to reveal its
phytochemicals and pharmacological activity. The plants are a
rich source of phytoconstituents. Searching new therapeutic
agents is a big challenge for the scientist of the present modern
era and plants are the biggest source of these agents. Screening
of plants for their pharmacological properties with the hope
of finding safe and effective agents is very essential. A large
number of synthetic compounds are available but due to their
environmental pollution and adverse effect on the human
body there use is restricted. To find the safe, effective, and
environmental friendly agent from a plant source, C. procera
is a plant that may present as effective one. In conclusion, the
literature on C. procera suggests a huge biological potential
of this plant. It is believed that the present manuscript may
be useful to provide additional information with regard to its
identification and in accordance to carry out further research
on its use in the treatment of various diseases.
REFERENCES
1. Sharma K, Kharb R, Kaur R. Pharmacognostical aspects of
Calotropis procera (Ait.) R.Br. Int J Pharm Bio Sci 2011;2:1-9.
2. Ahmed KK, Rana AC, Dixit VK. Calotropis species (Ascelpediaceae):
A comprehensive review. Pharmacogn Mag 2005;1:48-52.
3. Parrotta JA. Healing Plants of Peninsular India. Wallingford, UK
and New York: CAB International; 2001. p. 944.
4. Smith NM. Weeds of the wet-dry tropics of Australia - A field
guide. Environ Centre NT 2002;112:28-9.
5. Francis JK. U.S. Department of Agriculture, International
Institute of Tropical Forestry. University of Puerto Rico, PR
00936-4984; 1974.
6. Kleinschmidt HE, Johnson RW. Weeds of Queensland. Brisbane:
Goverment Printer; 1977. p. 469.
7. Anonymous. Himalaya Herbal Health Care. 2007b. Available
from: http://www.himalayahealthcare.com/herbfinder/h_
calotropis.htm.
8. Shinde SR, Ghatge RD, Mehetre SS. Comparative studies on the
growth and development of sandalwood tree in association with
different hosts. Indian J Forest 1993;162:165-6.
9. Verma R, Satsangi GP, Shrivastava JN. Ethno-medicinal profile of
different plant parts of Calotropis procera (Ait.) R.Br. Ethnobot.
Leafl 2010;14:721-42.
10. Saber H, Maharan GH, Rizkallah MM. Sterols and pentacyclic
triterpenes of Calotropis procera. Bull Fac Pharm 1969;7:91-104.
11. Tiwari KP, Masood M, Rathore S, Minocha PK. Study of
anthocyanins from the flowers of some medicinal plants. Vijnana
Parishad Anusandhan Patrika 1978;21:177-8.
12. Carruthers B, Griffiths DJ, Home V, Williams LR. Hydrocarbons
from Calotropis procera in Northern Australia. Biomass
1984;4:275-82.
13. Shukla OP, Krishnamurthy CR. Properties and partial purification
of bacteriolytic enzyme from the latex of Calotropis procera
(Madar). J Sci Ind Res 1961;20:109-12.
14. Chatterjee A, Pakrashi SC. The Treatise on Indian Medicinal
Plants. Vol. 4. New Delhi: Publications and Information
Directorate, C.S.I.R.; 1995. p. 1-3.
15. Kaushik P, Dhiman AK. Medicinal Plants and Raw Drugs of India.
Deheradun: Shiva Offset Press; 1999. p. 358-60.
16. Chaudhari HN. Pharmacognostic studies on the leaf of Calotropis
gigantean R.Br. Ex Ait. Bull Bot Surv India 1961;3:171-3.
17. Sharma BM. Root systems of some desert plants in churu,
Rajasthan. Indian Forest 1968;94:240-6.
18. Sabrin RM, Ibrahim GA, Mohamed LA, Shaala LM, Banuls Y, Van
Goietsenoven G, et al. New ursane-type triterpenes from the root
bark of Calotropis procera. Phytochem Lett 2012;5:490-5.
19. Mittal A, Ali M. Diterpenic labdane galactofuranosides from roots
of Calotropis procera (Ait.) R. Br Indian JChem 2013;52:641-5.
20. Dwivedi B, Singh A, Mishra S, Singh R, Pant P, Thakur LK, et al.
Evaluation of phytochemical constituents by gas chromatography-
mass spectroscopy & HPTLC of Calotropis procera. World J Pharm
Res 2014;3:708-715.
21. Ibrahim SR, Mohamed GA, Shaala LA, Moreno L, Banuls Y, Kiss R,
et al. Proceraside A, a new cardiac glycoside from the root barks
of Calotropis procera with in vitro anticancer effects. Nat Prod Res
2014;28:1322-7.
22. Mohamed NH, Liu M, Abdel-Mageed WM, Alwahibi LH, Dai H,
Ismail MA, et al. Cytotoxic cardenolides from the latex of
Calotropis procera. Bioorg Med Chem Lett 2015;25:4615-20.
23. Sweidan NI, Abu Zarga MH. Two novel cardenolides from
Calotropis procera. J Asian Nat Prod Res 2015;17:900-7.
24. Ibrahim SR, Mohamed GA, Shaala LA, Banuls LM, Kiss R,
Youssef DT. Calotroposides H-N, new cytotoxic oxypregnane
oligoglycosides from the root bark of Calotropis procera. Steroids
2015;96:63-72.
25. Meena AK, Yadav AK, Niranjan US, Singh B, Nagariya AK,
Sharma K, et al. A review on Calotropis procera Linn and its
ethnobotany, phytochemical, pharmacological profile. Drug
Invent Today 2010;2:185-90.
26. Quazi S, Mathur K, Arora S. Calotropis procera: An overview of its
phytochemistry and pharmacology. Indian J Drugs 2013;1:63-9.

Parihar and Balekar: C. Procera: A review
130 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
27. Gupta S, Gupta B, Kapoor K, Sharma P. Ethnopharmocological
potential of Calotropis procera: An overview. Int Res J Pharm
2012;3:19-22.
28. Khairnar AK, Bhamare SR, Bhamare HP. Calotropis procera: An
ethnopharmacological update. Adv Res Pharm Biol 2012;2:142-56.
29. Alam P, Ali M. Phytochemical investigation of Calotropis procera
Ait roots. Indian J Chem 2009;48:443-6.
30. Cavalcante GS, de Morais SM, Andre WP, Ribeiro WL,
Rodrigues AL, De Lira FC, et al. Chemical composition and in
vitro activity of Calotropis procera (Ait.) latex on Haemonchus
contortus. Vet Parasitol 2016;226:22-5.
31. Sayed Ael-D, Mohamed NH, Ismail MA, Abdel-Mageed WM,
Shoreit A. Antioxidant and antiapoptotic activities of Calotropis
procera latex on Catfish (Clarias gariepinus) exposed to toxic
4-nonylphenol. Ecotoxicol Environ Saf 2016;128:189-94.
32. Tsala DE, Nga N, Thiery BN, Bienvenue MT, Theophile D.
Evaluation of the antioxidant activity and the healing action of
the ethanol extract of Calotropis procera bark against surgical
wounds. J Intercult Ethnopharmacol 2015;4:64-9.
33. Vaiyapuri PS, Ali AA, Mohammad AA, Kandhavelu J,
Kandhavelu M. Time lapse microscopy observation of cellular
structural changes and image analysis of drug treated cancer
cells to characterize the cellular heterogeneity. Environ Toxicol
2015;30:724-34.
34. Gurung AB, Ali MA, Bhattacharjee A, AbulFarah M, Al-Hemaid F,
Abou-Tarboush FM. Molecular docking of the anticancer bioactive
compound proceraside with macromolecules involved in the cell
cycle and DNA replication. Genet Mol Res 2016;15(2):1-7.
35. Oloumi H. Phytochemistry and ethno-pharmaceutics of Calotropis
procera. Ethno Pharm Prod 2014;1:1-8.
36. Chaudhary P, Ramos MV, Vasconcelos Mda S, Kumar VL.
Protective effect of high molecular weight protein sub-fraction of
Calotropis procera latex in monoarthritic rats. Pharmacogn Mag
2016;12:147-51.
37. Ramos V, Aguiar VC, Melo VM, Mesquita RO, Silvestre PP,
Oliveira JS, et al. Immunological and allergenic responses
induced by latex fractions of Calotropis procera (Ait.) R.Br.
J Ethnopharmacol 2007;111:115-22.
38. Parihar G, Balekar N. Immunomodulating potential of Calotropis
procera (Ait.) root bark ethanolic extract on experimental animal.
J Adv Pharm Educ Res 2014;4:268-76.
39. Nascimento DC, Ralph MT, Batista JE, Silva DM, Gomes-
Filho MA, Alencar NM, et al. Latex protein extracts from
Calotropis procera with immunomodulatory properties protect
against experimental infections with Listeria monocytogenes.
Phytomed 2016;23(7):745-53.
40. Aliyu RM, Abubakar MB, Kasarawa AB, Dabai YU, Lawal N,
Bello MB, et al. Efficacy and phytochemical analysis of latex of
Calotropis procera against selected dermatophytes. J Intercult
Ethnopharmacol 2015;4:314-7.
41. Van QE, Simon G, André A, Dewelle J, El Yazidi M, Bruyneel F,
et al. Identification of a novel cardenolide (2’’-oxovoruscharin)
from Calotropis procera and the hemisynthesis of novel derivatives
displaying potent in vitro antitumor activities and high in vivo
tolerance: Structure activity relationship analyses. J Med Chem
2005;10:849-56.
42. Wang ZN, Wang MY, Mei WL, Han Z, Dai HF. A new cytotoxic
pregnanone from Calotropis gigantea. Molecules 2008;13:3033-9.
43. Mittal A, Ali M. Terpenoid glycosides from the roots of Calotropis
procera (Ait.) R.Br. Der Pharm Lett 2012;4:307-13.
44. Ibrahim SR, Mohamed GA, Shaala LA, Banuls LM,
Goietsenoven GV, Kiss R, et al. New ursane-type triterpenes from
the root bark of Calotropis procera. Phytochem Lett 2012;5:490-5.
45. Chundattu SJ, Agrawal VK, Ganesh N. Phytochemical investigation
of Calotropis procera. Arab J Chem 2011; In press. Available
from: http://www.dx.doi.org/10.1016/j.arabjc.2011.03.011.
46. Mittal A, Ali M. Acyclic diterpenic constituents from the roots of
Calotropis procera (Ait.) R.Br. J Saudi Chem Soc 2011;19:59-63.
47. Khasawneh MA, Elwy HM, Fawzi NM, Hamza AA,
Chevidenkandy AR, Hassan AH. Anti oxidant activity,
lipoxygenase inhibitory effect and polyphenolic compounds from
Calotropis procera (Ait.) R.Br. Res J Phytochem 2011;5:80-8.
48. Doshi HV, Parabia FM, Sheth FK, Kothari IL, Parabia MH, Ray A.
Phytochemical analysis revealing the presence of two new
compounds from the latex of Calotropis procera (Ait.) R.Br. Int J
Plant Res 2012;2:28-30.
49. Mohamed MA, Hamed MM, Ahmed WS, Abdou AM. Antioxidant
and cytotoxic flavonols from Calotropis procera. Z Naturforsch
2011;66:547-54.
50. Gallegos Olea RS, Oliveira AV, Silveira LM, Silveira ER.
Organic carbonate from Calotropis procera leaves. Fitoterapia
2002;73:263-5.
51. Heneidak S, Grayer RJ, Kite GC, Monique SJ. Flavonoid
glycosides from Egyptian species of the tribe Asclepiadeae
(Apocynaceae, subfamily Asclepiadoideae). Simmonds Biochem
Syst Ecol 2006;34:575-84.
52. Kumar VL, Padhy BM. Protective effect of aqueous suspension of
dried latex of Calotropis procera against oxidative stress and renal
damage in diabetic rats. Biocell. 2011;35:63-9.
53. Patil RA, Makwana AB. Anti-hyperbilirubinemic and wound
healing activity of aqueous extract of Calotropis procera leaves in
wistar rats. Indian J Pharmacol 2015;47:398-402.
54. Agarwal K, Varma R. Ethnobotanical study of antilithic plants of
Bhopal district. J Ethnopharmacol 2015;174:17-24.
55. Muraina A, Adaudi AO, Mamman M, Kazeem HM, Picard J,
McGaw LJ, et al. Antimycoplasmal activity of some plant
species from Northern Nigeria compared to the currently used
therapeutic agent. Pharm Biol 2010;48:1103-7.
56. Oliveira RS, Figueiredo IS, Freitas LB, Pinheiro RS, Brito GA,
Alencar NM, et al. Inflammation induced by phytomodulatory
proteins from the latex of Calotropis procera (Asclepiadaceae)
protects against Salmonella infection in a murine model of
typhoid fever. Inflamm Res 2012;61:689-98.
57. Dieye AM, Tidjani MA, Diouf A, Bassene E, Faye B. Senegalese
pharmacopoeia: Study of acute toxicity and antitussive
activity of Calotropis procera AIT (Asclepiadaceae). Dakar Med
1993;38:69-72.
58. Ramos V, Viana CA, Silva AF, Freitas CD, Figueiredo IS, Oliveira RS,
et al. Proteins derived from latex of Calotropis procera maintain
coagulation homeostasis in septic mice and exhibit thrombin and
plasmin-like activities. Naunyn Schmiedebergs Arch Pharmacol
2012;385:455-63.
59. Malabade R, Taranalli AD. Calotropis procera: A potential
cognition enhancer in scopolamine and electroconvulsive shock-
induced amnesia in rats. Indian J Pharmacol 2015;47:419-24.
60. Moustafa AM, Ahmed SH, Nabil ZI, Hussein AA, Omran MA.
Extraction and phytochemical investigation of Calotropis procera:
Effect of plant extracts on the activity of diverse muscles. Pharm
Biol 2010;48:1080-190.
61. Freitas D, Oliveira JS, Miranda MR, Macedo NM, Sales MP, Villas-
Boas LA, et al. Enzymatic activities and protein profile of latex
from Calotropis procera. Plant Physiol Biochem 2007;45:781-9.
62. Freitas AP, Bitencourt FS, Brito GA, de Alencar NM, Ribeiro RA,
Lima-Júnior RC, et al. Protein fraction of Calotropis procera latex
protects against 5-fluorouracil-induced oral mucositis associated
with down regulation of pivotal pro-inflammatory mediators.
Naunyn Schmiedebergs Arch Pharmacol 2012;385:981-90.
63. Singhal A, Kumar VL. Effect of aqueous suspension of dried
latex of Calotropis procera on hepatorenal functions in rat.
J Ethnopharmacol 2009;122:172-4.
64. Kumar VL, Sehgal R. Calotropis procera latex-induced
inflammatory hyperalgesia - effect of bradyzide and morphine.
Auton Autacoid Pharmacol 2007;27:143-9.
65. Akinloye K, Abatan MO, Onwuka SK, Alaka OO, Oke BO. Lipolytic

Parihar and Balekar: C. Procera: A review
131 TJPS 2016, 40 (3): 115-131
http://www.tjps.pharm.chula.ac.th
effect of Calotropis procera in the skin of wistar rats. Afr J Biomed
Res 2001;4:143-5.
66. Abdel-Mageed WM, Mohamed NH, Liu M, El-Gamal AA,
Basudan OA, Ismail MA, et al. Lipoxygenase inhibitors from the
latex of Calotropis procera. Arch Pharm Res 2016.
67. Khatter A. Morphogenetic abnormalities of Musca domestica
vicina induced by glycosidic groups from Calotropis procera
plant. Life Sci J 2012;9:1-7.
68. Rahman M, Islam W. Effect of acetonic extracts of Calotropis
procera R Br.in (Ait.) on reproductive potential of flat grain
beetle Cryptolestes pusillus (Schon.) (Coleoptera: Cucujidae).
Bangladesh J Sci Ind Res 2007;42:157-62.
