
Example: Assembling a Clinical Research and/or Clinical Trial Statement of Work
NOTE: This document is meant to provide recommendations for the preparation of a Statement of Work (SOW) associated with a Clinical Trial/Clinical
Research study. Please refer to the Funding Opportunity Announcement (FOA) and General Application Instructions for any specific SOW
requirements, including key regulatory milestones.
The SOW is used by the Congressionally Directed Medical Research Programs (CDMRP) to assess progress in completion of the scope of the work
outlined in the proposal. It serves as the synopsis of the entire project. During the entire period of performance, CDMRP will refer to this document
to assess scientific progress and success. The SOW should provide sufficient detail that upon reading, an individual unfamiliar with the project can
have a general understanding of the intent and approaches without referring to the proposal. However, directly copying narrative from the proposal
is not recommended.
Please consider the points below and include the following information (where applicable) when drafting your SOW:
1. Date your SOW according to when it was written/submitted/last edited.
2. List all study sites, including addresses with country where the work will be performed, that are receiving Department of Defense (DOD) funds
for the proposed project to include: partnering Principal Investigators (PIs), sub-awardees, or any other site where DOD-funded work is being
performed. If conducting a multisite cooperative clinical study within the United States identify the site that will be the central single
Institutional Review Board (IRB) of record.
3. Provide the specific aims as listed in the proposal.
4. Under each Specific Aim list associated tasks and subtasks. Please use 1-2 concise statements to describe all key experiments from the
proposal in the SOW. Detailed methodology is not necessary; state the goal of the task/subtask and then provide general types of
experiment(s) that will be used to achieve that goal (Example: Assess RNA expression of XYZ).
5. Next to each task/subtask indicate the study site and key personnel that is responsible for completion by listing the initials of the key personnel
in the appropriate column.
6. Provide a cohesive timeline that covers the entire period of performance and indicates the months during which each task is expected to be
performed. For example, Major Task 1 to occur in Months 1-3, Major Task 2 to occur in Months 2-6.
7. If conducting a study utilizing a drug or device for an indication not currently approved by the U.S. Food and Drug Administration (FDA) include
subtasks and milestones associated with Investigational New Drug (IND) or Investigational Device Exemption (IDE) submission and notification
to proceed.
8. If your study includes the clinical evaluation of an investigational product at international sites, include subtasks and milestones related to
the application for approval to proceed from the relevant national regulatory agency of the host country(ies).
9. If conducting a collaborative study with the Military Health System (MHS), a Military Treatment Facility (MTF), or if your research involves
access to active duty military patient populations and/or Department of Defense (DOD) resources and databases consider additional
agreements that need to be established before the study can be conducted. Include subtasks associated with the establishments of
Cooperative Research and Development Agreements (CRADAs), Memoranda of Understanding (MOU), Memoranda of Agreement (MOA),
Data Sharing Agreements (DSAs), Material Transfer Agreements (MTAs) or Clinical Trial Agreements (CTAs).

10. Clinical trials/clinical research studies are subject to United States Army Medical Research & Development Command (USAMRDC) Office of
Human Research Oversight (OHRO) review following IRB approval. Includes tasks/subtasks associated with obtaining the initial IRB approval
and secondary OHRO review for each study that is to be conducted.
o If conducting a multisite cooperative clinical study, include subtasks for review and approval of the master protocol and consent
form by the central IRB of record. Include milestones for implementation and approval of satellite study locations. Subtasks
associated with review and approval by the USAMRDC OHRO should follow IRB approval.
NOTE: OHRO approval must be in place before using DOD funds to conduct any research involving human subjects/material as outlined
in OHRO’s guidance. Please see: https://mrdc.health.mil/index.cfm/collaborate/research_protections/hrpo for more details.
11. The quarterly projected subject enrollment goals should be summarized on a participating site basis in a table at the end of the SOW. If
proposing multiple clinical studies separate tables should be prepared for each study.
12. If conducting a clinical trial, include a subtask for study registration with the National Institutes of Health (NIH) clinical trial registry
www.clinicaltrials.gov.
13. If applicable, include required study initiation milestones as outlined in the FOA.
14. If performing research involving animals, include a subtask for local Institutional Animal Care and Use Committee (IACUC) and USAMRDC
Animal Care and Use Review Office (ACURO) approval. Make sure to update the timeline of any tasks involving animal research so that they
do not overlap with the time period designated for ACURO review/approval.
NOTE: ACURO approval must in be place before using DOD funds for the proposed animal work. Please see:
https://mrdc.health.mil/index.cfm/collaborate/research_protections/acuro for more details.
15. If performing research involving human anatomical substances, cadavers, or human cells (excluding commercially available cell lines) include
a subtask for local IRB and USAMRDC OHRO. Make sure to update the timeline of any tasks involving these resources so that they do not
overlap with the time period designated for OHRO review/approval.
NOTE: OHRO approval must in be place before using DOD funds for the proposed studies. Please see:
https://mrdc.health.mil/index.cfm/collaborate/research_protections/hrpo for more details.
16. For all tasks/subtasks involving animals or human anatomical substances/human cells (including cell lines), please indicate the species/strain,
source, sex and numbers required as appropriate. A table containing this information can also be included at the end of the SOW.
17. Define all abbreviations upon first use or include an abbreviation list at the end of the SOW.

The below fictitious SOW is completed and is meant to serve as an example of how to structure a clinical research SOW. All tasks/subtasks and
milestones should be modified and adjusted as appropriate to align with the proposal. A blank SOW template is available for download on
eBRAP (https://ebrap.org/eBRAP/public/Program.htm).
STATEMENT OF WORK – Month/Day/Year
PROPOSED START DATE Month/Day/Year
Site 1:
State University (State Univ.)
Site 2:
Air Force Hall Medical Center
100 Circle Circle
100 Drive Drive
Town, TX 10000
United States
City, CO 30000
United States
PI: John Coltrane, PhD (JC)
Coordinator: Billy Holiday, PhD (BH)
Data Core Manager: Charlie Parker, PhD (CP)
Central IRB
PI: Dave Brubeck, PhD (DB)
Coordinator: Dizzy Gillespie, PhD (DG)
Site 3:
Army Medical Center
Site 4:
Veteran's Administration Medical Center
200 Street Street
300 Way Way
Town, GA 40000
United States
City, NY 50000
United States
PI: Miles Davis, PhD (MD)
PI: Duke Ellington, PhD (DE)
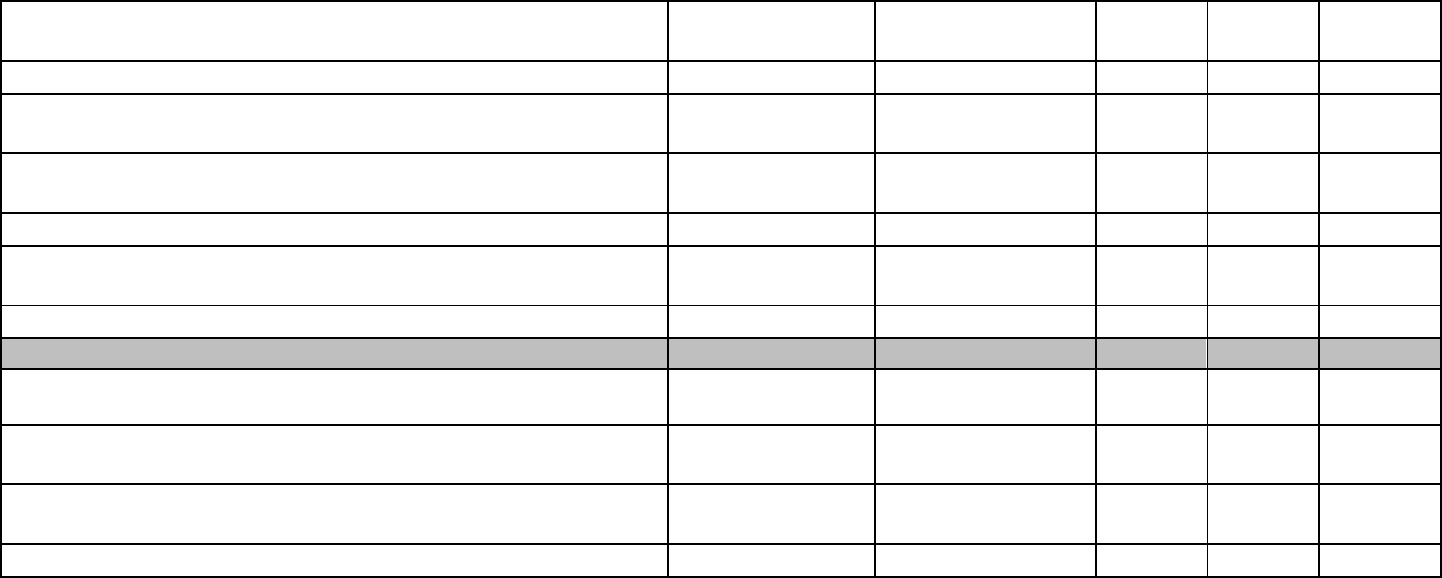
Study 1 Specific Aims 1&2: (1)To determine the adaptations of the
standard Jazz protocol that are needed to implement an outdoor-
based treatment in outdoor care; (2) To develop a model of care for
musical exposure therapy treatment of PTSD in outdoor care settings
including treatment length.
Timeline
Research Sites
Months
State Univ.
AFHMC
AMC
VA
Major Task 1: Adapt Standard Jazz Protocol for Outdoor Care Setting
Subtask 1: Prepare Regulatory Documents and Research Protocol for Study 1
If Applicable, coordinate with Sites for CRADA, MOU, MOA, and/or
DSA submission
1-3
JC/BH
DG
MD
DE
If Applicable, coordinate with Sites for MTAs or CTAs submission
1-3
JC/BH
DG
MD
DE
If Applicable, coordinate with Sites for NDAs.
1-3
JC/BH
DG
MD
DE
If applicable, indicate time required for submission and exemption of
an IND application or an IDE application to the FDA
1-3
JC/BH
DG
MD
DE

Refine eligibility criteria, exclusion criteria, screening protocol
1-3
JC/BH
DB
MD
DE
Finalize consent form & human subjects protocol
1-3
JC/BH
DB
MD
DE
Coordinate with Sites for IRB protocol submission
1-3
JC/BH
DG
MD
DE
Coordinate with Sites for State University IRB review
1-6
JC/BH
Coordinate with Sites for USAMRDC review (OHARO/OHRO)
1-6
JC/BH
DG
MD
DE
Clinicaltrial.gov registration
6
JC/BH
Submit amendments, adverse events and protocol deviations as
needed
As Needed
JC/BH
DG
MD
DE
Coordinate with Sites for annual IRB report for continuing review
Annually
JC/BH
DG
MD
DE
Milestone Achieved: Local IRB approval at AFHMC, AMC #1, and
VA#1
3
JC/BH
DG
MD
DE
Milestone Achieved: OHRO approval for all protocols and local IRB
approval through State Univ.
6
JC/BH
DG
MD
DE
Major Task 2: Coordinate Study Staff for Clinical Trials
Subtask1: Hiring and Training of Study Staff
Coordinate with Sites for job descriptions design
1-4
JC/BH
DG
MD
DE
Advertise and interview for project related staff
4-8
JC/BH
DG
MD
DE
Coordinate for space allocation for new staff
1-7
DG
MD
DE
Coordinate with Sites for Independent Evaluators hiring and
trainings
5-11
JC/BH
DG
MD
DE
Coordinate with Sites for training Independent Evaluators until 100%
concordance
8-11
JC/BH
DG
MD
DE
Milestone Achieved: Research staff trained
8-11
JC/BH
DG
MD
DE
Subtask 2: Facilitate and Coordinate with Sites for hiring, training,
supervision and fidelity checks as needed for attrition
8-20
JC/BH
DG
MD
DE
Coordinate with Sites for training Independent Evaluators to
maintain 100% concordance
8-20
JC/BH
DG
MD
DE
Milestone Achieved: Maintained trained and available Independent
Evaluators throughout duration of both clinical trials
8-20
JC/BH
DG
MD
DE
Study 1 Specific Aims 3, 4 & 5: (3) To develop a manualized outdoor
care treatment protocol for combat-related PTSD; (4) To determine
the level of PTSD symptom severity that is most suitable for long
outdoor-based jazz treatments in outdoor care; and (5) To conduct a
small (N = 35) clinical replication series study of the effectiveness of

Jazz therapy for the treatment of PTSD symptoms in outdoor care
settings.
Major Task 3: Prepare Research Protocol for Study 2
Subtask 1: Refine research protocol for study 2 based on findings of
study 1
20-25
JC/BH
DG
MD
DE
Milestone Achieved: protocol for study 2 developed
25-30
JC/BH
DG
MD
DE
Major Task 4: Participant Recruitment, Therapy, Participant Evaluation
Subtask 1: Study 1, Pilot Study
Coordinate with Sites for flow chart for all study steps, web data
collection and database requirements
4-8
JC/BH/CP
DG
MD
DE
Finalize assessment measurements
1-4
JC/BH/CP
DG
MD
DE
Milestone Achieved: 1st participant consented, screened and enrolled
7-30
DG
MD
DE
Milestone Achieved: Study 1 begins
7-30
DG
MD
DE
Begin subject recruitment
7-30
DG
MD
DE
Participants complete assigned treatment regimen over 12 weeks - 4
session outdoor care PTSD protocol N=35
7-30
DG
N=17
MD
N=7
DE
N=11
Complete follow-up assessments 3 months after completion of the
Outdoor Jazz treatment.
10-33
DB
MD
DE
Milestone Achieved: Report findings from 3 month follow-up
assessments
34-36
DG
MD
DE
Subtask 2: Determine modifications of Outdoor protocol that are
needed to implement Outdoor-based Jazz treatment in outdoor care
1-4
JC/BH
DB
MD
DE
Develop a model of care for outdoor therapy treatment of PTSD in
outdoor care
1-4
JC/BH/CP
DB
MD
DE
Develop a manualized outdoor care treatment protocol for combat-
related PTSD
1-4
JC/BH/CP
DB
MD
DE
Milestone Achieved: Treatment protocol finalized
5-7
JC/BH/CP
DG
MD
DE
Study 2 Specific Aim: To conduct a small randomized clinical trial (N =
60) to evaluate the efficacy of Outdoor Jazz therapy for OIF/OEF
veterans in outdoor care as compared to treatment as usual.
Major Task 5: Randomized Controlled Trial, Study 2
Subtask 1: Conduct Study, Report Findings
Analyze, measure and determine the appropriate level of PTSD
symptom severity that is most suitable for outdoor-based
treatments in outdoor care
31-36
DG
MD
DE
Milestone Achieved: 1st participant consented, screened and enrolled
in study 2
37-50
DG
MD
Milestone Achieved: Study 2 begins
37-50
DG
MD
Study 2 - Screen potential participants at outdoor care providers
using outdoor care PTSD process in Study 1 and consent (N=60)
37-50
DG
N=30
MD
N=30
DE
N=0
Study 2 - evaluate and assign participants to one of the two
randomized groups
37-50
DG
MD
Study 2 - assess all participants at the 3, 6, and 12 month timeframe
37-50
DB
MD
Study 2 - evaluate and measure the efficacy of Outdoor Jazz therapy-
PTSD for OIF/OEF veterans in outdoor care
37-50
DB
MD
Milestone Achieved: Report findings from overall studies
51-59
JC/BH/CP
DG
MD
DE
Major Task 6: Data Analysis
Subtask 1: Coordinate with Sites & Data Core for monitoring data
collection rates and data quality
51-59
CP
DG
MD
DE
Perform all analyses according to specifications, share output and
finding with all investigators
51-59
CP
DG
MD
DE
Work with data core and dissemination of findings (abstracts,
presentation, publications, DOD)
51-59
CP
DG
MD
DE
Milestone Achieved: Report results from data analyses
51-59
JC
DG
MD
DE
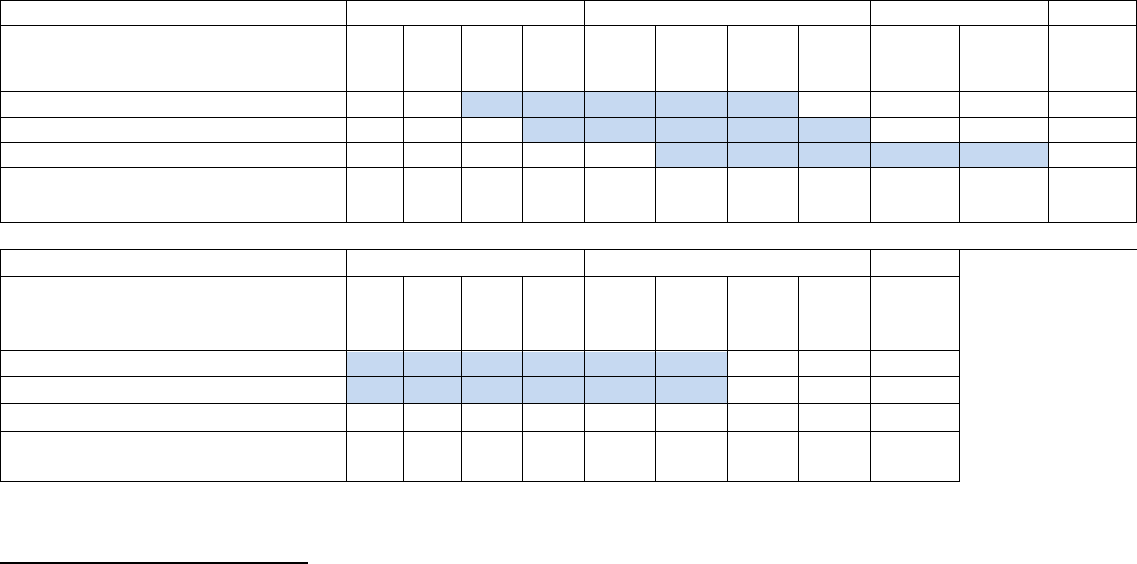
Projected Quarterly Enrollment
Note: The Government reserves the right to request a revised SOW format and/or additional information.
Abbreviations List (if necessary)
Year 1
Year 2
Year 3
Total
Target Enrollment Pilot Study (#1)
(per quarter)
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
AFHMC
-
-
5
5
4
1
2
0
0
0
17
AMC #1
-
-
0
2
1
3
0
1
0
0
7
VA #1
-
-
0
0
0
1
2
4
3
1
11
Target Enrollment
(cumulative)
-
-
5
7
5
5
4
5
3
1
35
Year 3
Year 4
Total
Target Enrollment Randomized Control
Study (#2)
(per quarter)
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
AFHMC
3
3
6
6
6
6
0
0
30
AMC #1
3
3
6
6
6
6
0
0
30
VA #1
0
0
0
0
0
0
0
0
0
Target Enrollment
(cumulative)
6
6
12
12
12
12
0
0
60
